Product List Background C5b Functional Service
Background
C5b is the first complement component to initiate MAC formation. As the three pathways of complement produce proteolytic enzyme complexes (C3/C5 convertases) which are bound to the target surface, these enzymes cleave a peptide bond in the larger alpha chain of C5 leading to the release of C5a and activating C5b. The C5b fragment is intrinsically labile and if not binds to C6, it will become inactive within 2 minutes. Furthermore, C5b,6 complex remains bound to the C3/C5 convertase until it binds a single C7 molecule. And in the absence of the C7 protein, C5b,6 is released into the fluid phase. The C5b67 complex is rendered lipophilic and is drawn into the phospholipid bilayer of the cell membrane or viral envelope. During complement activation, some C5b,6 diffuses away from the target cell and may, after combining with C7, enter the membrane of a nearby cell.
C5b–C7 binds to the plasma membrane via a hydrophobic site on C7 becoming exposed so that it can be inserted into the lipid bilayer of the cell membrane. After that, C8 binds to the C5b-C7 complex and C9 binds to C8 within the C5b-C8 complex, resulting in C9 undergoing a conformational change that causes the exposure of a hydrophobic domain that made C9 insertion by the membrane lipid bilayer.
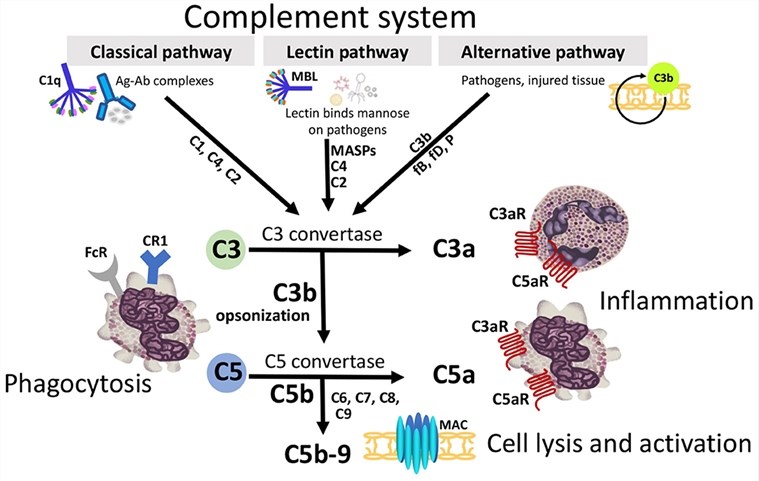 Fig.1 Diagram illustrating how the convergence of three distinct extracellular complement activation pathways leads to a shared terminal pathway.1, 3
Fig.1 Diagram illustrating how the convergence of three distinct extracellular complement activation pathways leads to a shared terminal pathway.1, 3
C5b Functional Service
Creative Biolabs offers a comprehensive collection of C5b-associated products, such as anti-C5b antibodies and ELISA kits for detecting C5b. These carefully designed reagents are essential in advancing research aimed at creating therapeutic solutions for numerous medical conditions.
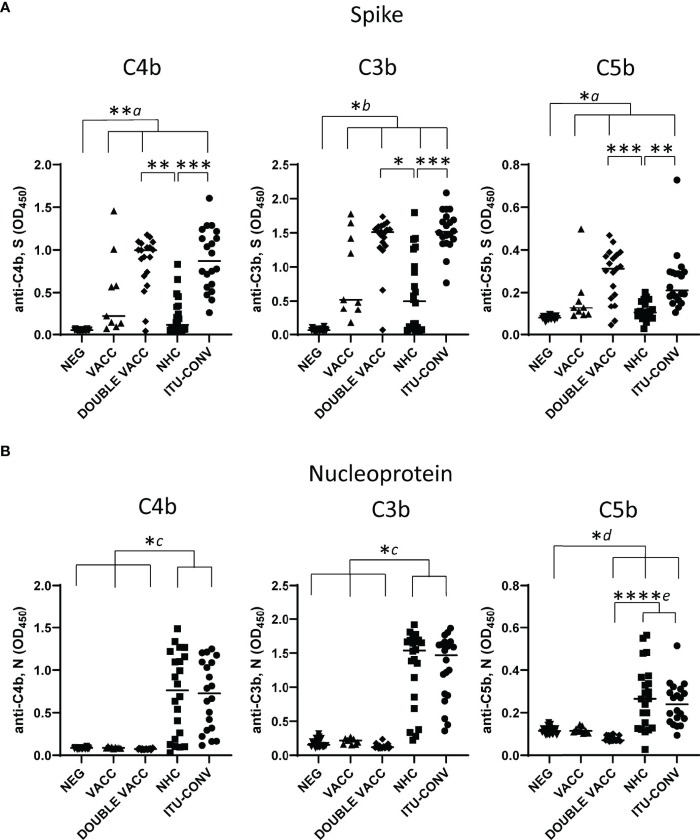 Fig.2 Variability in C4b, C3b, and C5b influenced by antigen type and subject condition.2, 3
Fig.2 Variability in C4b, C3b, and C5b influenced by antigen type and subject condition.2, 3
Researchers have identified antibodies against SARS-CoV-2 spike (S) and nucleocapsid (N) proteins, typically present in severe COVID-19 cases and after vaccination. These antibodies can trigger the classical complement pathway, which may have varied pathological or protective roles during different infection phases. The study evaluated antibody-dependent complement activation using ELISA to measure complement protein binding (C1q) and the deposition of C4b, C3b, and C5b on S and N proteins. Variability was noted in downstream complement component detection, with consistent C3b-C5b detection linked to S in convalescent hospitalised subjects, unlike the NHC group. Conversely, complement deposition responses to N showed no differences between these groups. Thus, classical pathway activation by SARS-CoV-2 antibodies depends on disease status and targeted antigen.
Creative Biolabs offers a broad spectrum of tailored C5b-related functional services, encompassing thorough interaction evaluations and diverse expert analyses. These meticulously designed solutions aim to support clients in advancing their scientific research and clinical initiatives.
References
-
Girardi, Guillermina, et al. "Essential role of complement in pregnancy: from implantation to parturition and beyond." Frontiers in immunology 11 (2020): 1681.
-
Lamerton, Rachel E., et al. "SARS-CoV-2 spike-and nucleoprotein-specific antibodies induced after vaccination or infection promote classical complement activation." Frontiers in immunology 13 (2022): 838780.
-
Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig.1 Diagram illustrating how the convergence of three distinct extracellular complement activation pathways leads to a shared terminal pathway.1, 3
Fig.1 Diagram illustrating how the convergence of three distinct extracellular complement activation pathways leads to a shared terminal pathway.1, 3
 Fig.2 Variability in C4b, C3b, and C5b influenced by antigen type and subject condition.2, 3
Fig.2 Variability in C4b, C3b, and C5b influenced by antigen type and subject condition.2, 3
