The complement system has long been recognized as a central player in innate immunity, defending the host against infections while maintaining tissue homeostasis. Among its components, complement C3 stands as a pivotal node in the cascade, orchestrating a wide array of immune responses. Recent advancements in structural biology and molecular immunology uncover more nuanced insights into how modulation of this system can yield powerful therapeutic benefits, particularly in treating diseases marked by complement dysregulation.
One such groundbreaking study, published by Professor Feng Qian’s group at the School of Pharmaceutical Sciences, Tsinghua University, and featured in the Journal of Biological Chemistry, offers new perspectives on the structure-function relationship of C3 and highlights epitope-specific inhibition as a promising strategy for targeted immune modulation. This study reveals the complement biological effects of inhibiting different epitopes of complement C3 and provides valuable insights for the rational design of C3 inhibitors.
At Creative Biolabs, we are inspired by this innovative work, as it aligns with our commitment to advancing next-generation complement therapeutics. Let us delve deeper into this study and explore how epitope-specific targeting of C3 can shape the future of precision immunotherapy.
Understanding Complement C3: A Central Player in Immune Defense
The complement system is an essential component of the immune system and plays a key role in the maintenance of tissue homeostasis and immunosurveillance through modulation-mediated phagocytosis, immunomodulatory activity, and pathogen lysis. The complement cascade response is tightly regulated by multiple negative regulatory factors to prevent aberrant activation leading to host cell and tissue damage. Dysregulation and overactivation of the complement system are associated with a variety of diseases:
- Paroxysmal Nocturnal Hemoglobinuria (PNH)
- Geographic Atrophy (GA)
- Wet AMD (wAMD)
Complement C3 is the most abundant component in the complement system and is integral to all three complement activation pathways—classical, lectin, and alternative. Through its active fragments such as C3a and C3b, C3 contributes to pathogen opsonization, immune cell recruitment, and the formation of the membrane attack complex (MAC).
As the most abundant complement component in serum, C3 is a central component in the complement cascade reaction and is involved in the production of all complement activity products (C3a, C3b, C5a, C5b-9). Given C3’s central role, it is no surprise that this protein has become a primary target for therapeutic development.
C3 Inhibitors in Clinical Use and Trials
C3-targeting therapeutics have already made their mark in the clinic. Currently, a polyethylene glycolated peptide injection and an intravitreal injection have been approved for the treatment of PNH and GA, respectively, and the active ingredient of both is an anti-C3 cyclic peptide. In addition, more than a dozen C3-targeted drugs are currently in preclinical or clinical studies.
Yet not all C3 inhibitors achieve clinical success. For example, while Syfovre succeeded in Phase III trials for GA, the monoclonal antibody NGM621 failed to meet its efficacy endpoint in Phase II. These discrepancies raise critical questions: What determines the efficacy of a C3 inhibitor? Can we fine-tune targeting based on C3’s structural nuances?
The study addresses exactly these issues, providing a framework to understand how epitope-specific binding can differentially affect complement activity. Perhaps ideal C3 inhibitors need to bind key functional epitopes with high affinity. The complex structure-function relationship of C3 is a pressing issue in C3 drug design.
Epitope-Specific Inhibition: A Novel Strategy to Modulate C3
The research team engineered three model inhibitors (MIs) targeting distinct epitopes on the C3 molecule:
- MI1/MI2: Bind to the MG4/MG5 domains of C3/C3b
- MI3: Targets the C345C domain of C3
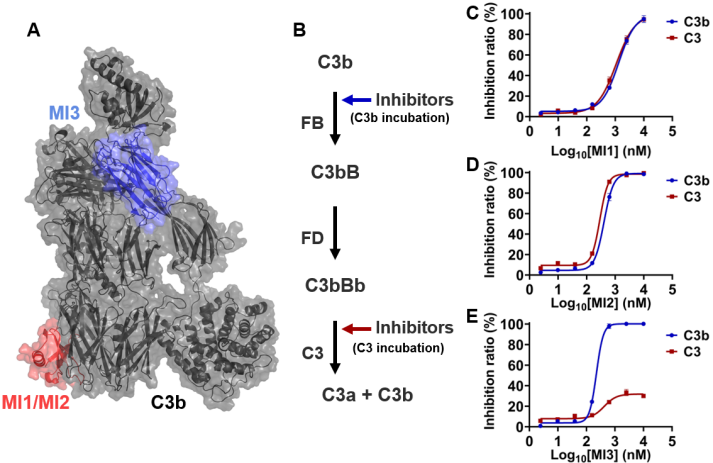
Fig. 1 Binding epitopes and inhibition mechanisms of C3 model inhibitors.1,2
By adding model molecules at different steps of the complement cascade reaction, the model molecules produced different inhibitory effects. The model molecule MI1/2 inhibited C3 cleavage when added at different reaction steps, whereas the model molecule MI3 produced C3 cleavage inhibitory activity only by inhibiting the step at which C3b binds to FB. Through a series of biochemical assays, the team revealed:
- MI1/MI2 exhibit strong inhibition across both alternative (AP) and classical (CP) pathways by preventing the formation of C3 convertases (C3bBb and C4bC2a).
- MI3 specifically blocks the alternative pathway by inhibiting the assembly of the C3 convertase C3bBb.

Fig. 2 Complement inhibition process targeting the C345C structural domain and the MG4/MG5 structural domain in C3.1,2
These findings underscore that the “epitope targeted by the inhibitor significantly influences the inhibitory profile”, highlighting the importance of precise epitope mapping in drug design.
Implications for Drug Development
- Rational design of C3 inhibitors: Structural insights allow developers to design inhibitors with tailored activity across specific pathways.
- Improved efficacy and safety: Selective inhibition may reduce off-target effects and allow more nuanced control over immune responses.
- Personalized medicine potential: Epitope-targeting strategies may align with patient-specific complement profiles and disease states.
At Creative Biolabs, we understand the complexities of designing complement-targeted therapies. That’s why we offer comprehensive complement therapeutic development services, including:
- Complement protein
- Complement functional assay
- Therapeutic antibody development
- Complement component inhibitor development
Explore our complement therapeutics solutions to see how we can accelerate your project.
Pushing the Boundaries: What’s Next?
- Investigate additional epitopes across C3 and its fragments (C3a, C3b)
- Develop bifunctional inhibitors that engage multiple domains
- Use AI-driven structure prediction to identify novel inhibitory hotspots
Creative Biolabs is proud to support such innovation with cutting-edge drug discovery platforms and biomolecular engineering tools. Together, we can unlock the full potential of the complement system in medicine.
Conclusion
The study marks a significant step forward in our understanding of complement biology. By showing that not all C3 inhibitors are created equal and that epitope-specific targeting can lead to vastly different therapeutic outcomes, the researchers have provided a critical roadmap for future drug development.
As a trusted partner in immunotherapy innovation, Creative Biolabs is here to support your journey from discovery to preclinic. Whether you’re developing novel complement inhibitors, performing structural analysis, or exploring epitope-specific binding, we have the expertise and resources to help you succeed.
References
- Chen, Zhidong, et al.“Modulating the complement system through epitope-specific inhibition by complement C3 inhibitors.” Journal of Biological Chemistry (2025): 108250. https://doi.org/10.1016/j.jbc.2025.108250
- Distributed under Open Access license CC BY 4.0, without modification.
