Exosomes
Exosomes are single-layer membrane vesicles secreted by cells with a diameter of 30-200 nm, rich in proteins, lipids, nucleic acids, and carbohydrate complexes, and have obvious molecular heterogeneity. The production of exosomes is a protein quality control mechanism with various biological activities, which can remodel the extracellular matrix, carry out intercellular signal and molecular transmission, and have links with development, immunity, tissue homeostasis, cancer and neurodegeneration Diseases and other aspects of human health are closely related. In addition, viruses can also use exosomes to assemble virus particles, or use exosomes to generate immune escape. At present, based on the relevant characteristics of exosomes, they have been used as therapeutic tools for various diseases.
Early research and definition of exosomes
In the study of the related ATPase and nuclease in the secreted vesicles, considering that the secreted vesicles have certain physiological functions, Trams et al. defined vesicle secretion, and believed that the secretion of vesicles is the It has universal functions and is not limited to specific cell types, and divides vesicles into about 40 nm in diameter, exosomes with abundant cell-derived proteins, nucleic acids, lipids and sugar complexes, and exosomes larger than 500 nm microvesicles, in which the material composition of microvesicles is more similar to that of cells. The centrifugal force of these two kinds of vesicles is also different during the collection process, microvesicles are collected at 10,000 xg for 30 min, and exosomes are collected at 70,000-100,000 xg for 90-120 min.
In the 1960s, Bonucci and Anderson et al. discovered vesicles secreted by chondrocytes with a diameter of about 100 nm. In their early studies, it was believed that these vesicles initiated the formation of hydroxyapatite crystals and released from the cell membrane Formed and secreted by budding. At the same time, Wolf et al. also reported that platelets can release some small extracellular vesicles, which play an important role in blood coagulation. These studies were further confirmed in later experiments, and the new study also confirmed the role of osteogenic exosomes in the deposition of bone and teeth, and in cardiovascular diseases, the pathological calcification of exosomes in arteries and heart also played an important role.
In the 1980s, other studies related to exosomes were gradually carried out, including the study of extracellular enzymes by Trams et al., who found that there were exosomes in the semen derived from the prostate/epididymis, and this part of the exosomes was not only sperm Necessary for maturation and transfers prostate-derived proteins and lipids to the sperm membrane. In the early 1980s studies on the disappearance of transferrin receptors from the plasma membrane of mature reticulocytes revealed that exosome biogenesis is a mechanism for the quality control of plasma membrane proteins, which involves the transferrin Selective endocytosis of protein receptors from the plasma membrane, budding from the endosomal membrane into the endosomal lumen, and fusion with the plasma membrane.
In general, the previous research established four principles of exosome biology, which were also confirmed by subsequent research routes:
- First, exosomes are produced by budding from the plasma and endosomal membranes
- Second, exosomes can deliver signals and macromolecules to target cells
- Third, exosome biogenesis is a mechanism of protein quality control
- Fourth, exosome biology plays an important role in human health and disease
The structure and composition of exosomes
In addition to being produced by cell secretion, exosomes may also be formed by fragmentation and sealing caused by physical and mechanical forces. These exosome vesicles can be found in various biological fluids or tissue supernatants, including lipoprotein particles, RNA- Protein particles and protein aggregates, etc. The density of exosomes is about 1.1-1.2 g/ml, and the density of vesicles is affected by the ratio of protein and lipid, which varies greatly among different vesicles. Studies have demonstrated that the expression of a single exosomal cargo protein can lead to an increase in exosome density and cause significant changes in exosome size and shape. In addition, the density of exosomes is also affected by exosome metabolic pathways, such as hydroxyapatite crystallization of osteogenic exosomes. In short, the size, shape, and density of exosomes are determined by specific proteins, lipids, enzymes, and mineral contents. Therefore, the density of exosomes is changing, not a fixed physical and chemical characteristic.
The properties of exosomes are directly related to the purification method. As mentioned above, exosomes and microvesicles are traditionally defined based on differential centrifugation, with microvesicles obtained after 1–2 centrifugations at 10,000 × g for 30 min, and exosomes obtained by centrifugation at 10,000 × g. The supernatant was obtained by centrifugation at 70,000–100,000 × g for 90–120 minutes. These harvested particles contain more than just exosomes, and if the starting material is a biological fluid such as plasma, the particle may contain significant amounts of chylomicrons and other lipoprotein particles, especially if the subject has recently eaten. It may also contain exosomes, aggregates of large proteins, ribonucleoprotein (RNP) complexes, and small microsomes produced by mechanical shearing of the cell membrane. Due to the collection of many small vesicles due to physical factors such as centrifugal force and centrifuge tubes, some studies require further purification of exosomes by density gradient centrifugation, while others can be achieved by immunoaffinity purification using specific antibodies for specific exosome surface antigens to improve the purity of exosomes.
Exosomes contain a wide range of transmembrane proteins, lipid-anchored membrane proteins, peripheral-associated membrane proteins, and soluble proteins in the exosome lumen, as shown in Figure 1. Exosomes are not uniform in size and composition, and are rich in membrane-associated oligomeric proteins. Although not all of these proteins are shown in the figure, exosomes are rich in tetrapeptides, adhesion molecules, enzymes, scaffolds, and RNA binding. Proteins, RNA, DNA and complex glycans.
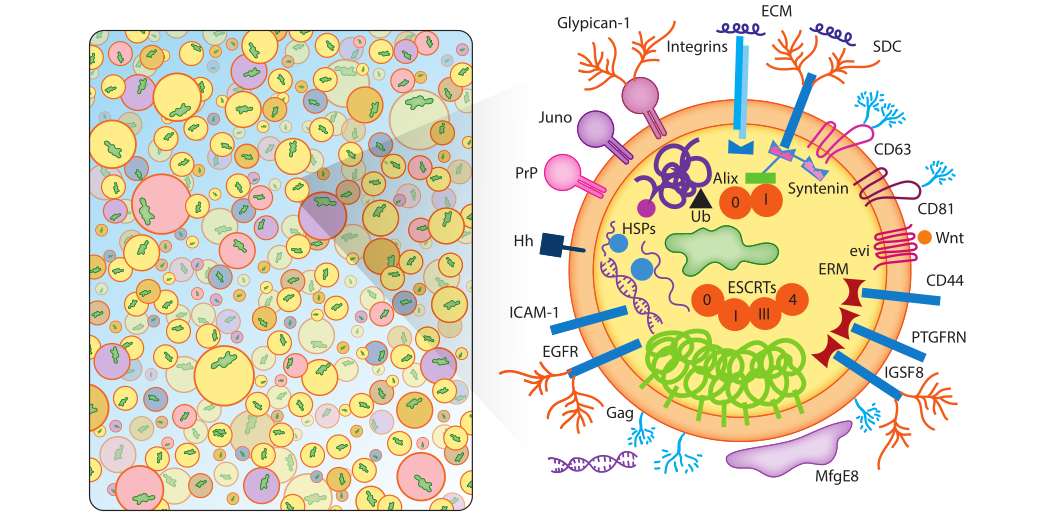
Figure 1: The list shown in Figure 1 is an illustrative list, not a comprehensive list of contents, and not one exosome will contain all or even most of the specified proteins.
a) Exosomal membrane proteins
Escola et al. demonstrated that certain tetrapeptide proteins (CD81, CD82, CD37, and CD63) were highly enriched in exosomes, whereas common plasma and lysosomal membrane proteins were not. Among them, CD81 is the most highly enriched protein in exosomes, mainly localized in the plasma membrane, while CD63 protein enriched in endosomes is the least enriched protein in exosomes. CD81 and CD63 have become the most commonly used exosome marker proteins, along with other tetrapeptides such as CD9. Tetrapeptides themselves are not catalytically active, but can promote the trafficking, function, stability, and oligomerization of other membrane proteins. Tetrapeptides mediate the action of other proteins. Exosomes are rich in tetrapeptide-related chaperones, major histocompatibility complex (MHC) class II proteins, immunoglobulin superfamily member 8 (IGSF8), intercellular Supported by evidence for adhesion molecule-1 (ICAM-1), integrins (SDC1-4), integrins, and many other proteins. The presence of integrins in exosomes is of particular biomedical importance as these proteins play key roles in the pre-migratory niche of cancer metastasis and the organotropism of tumor metastasis.
b) Lipid-anchored outer membrane proteins
The surface of exosomes contains a series of lipid-anchored protein modifications. These include: a number of C-terminal glycosylphosphatidylinositol-anchored proteins, ectonucleotidases CD39 and CD73, the sperm receptor Juno, complement inhibitory proteins CD55 and CD59, and the cellular prion protein PrPC and the amyloid isoform PrPSC. Lipid-anchored proteins on the surface of these exosomes play important roles in development and cancer and are partially anchored in the outer leaflet of the cell membrane by cholesterol.
c) Peripheral surface protein receptors
Exosomes carry peripheral surface receptors such as Wnt proteins involved in signaling, as well as integral membrane cargo receptors/sorting partners such as EVI. In addition, it also contains some tumor necrosis factor, transforming growth factor β, the first apoptosis signal FAS ligand and cytokines, etc., which further strengthens the view that exosomes serve as a variety of signaling platforms that transmit complex autocrine and paracrine signals. In addition, exosomes are also rich in extracellular matrix ECM proteins, which play an important role in signal transduction and adhesion.
d) Lipid-anchored inner membrane proteins
Exosomes contain an inner membrane rich in acylated lipid-anchored proteins such as prenylated small GTPases (Rabs, Ras, Rho, etc.), myristoylated signaling kinase (Src), and palmitoylated membrane proteins . The exosomal endothelium is rich in proteins that serve as scaffolds to connect other cross-linked proteins, chaperones, and lipids. ERMs, for example, cross-link plasma membrane proteins to cytoskeletal proteins and other scaffolds in response to signal-induced phosphorylation. Synapsin is another exosomal scaffolding factor that aggregates exosomal proteins through multiple protein-binding motifs. In addition to different scaffolding proteins, the inner membrane of exosomes is also rich in molecular chaperones that bind to aggregated and misfolded proteins, such as heat shock proteins HSP90, HSP20, HSP27, etc.
e) Enzyme
Exosomes are secreted, membranous, metabolically active platforms that, in addition to enzymes secreted by specific cells, also contain RNA editing enzymes, lipases, proteases, glycosyltransferases, and metabolic enzymes that can alter the content of exosomes substances, making exosomes a macromolecule storage pool different from cells. Exosomes derived from cancer cells are more potent due to the presence of mutated Ras proteins, receptors, hyaluronan synthase-3, and RNA interference-related activities. In addition, exosomes also contain key enzymes for energy metabolism.
f) Soluble proteins and inclusion bodies
Certain cargo proteins were highly enriched in exosomes, especially CD81, CD9, and CD63. Exosome biogenesis likely involves massive inclusions of cytoplasmic and membrane contents, with only a small fraction of exosomal proteins showing signs of active sorting. Under such a model, soluble proteins might be rarely incorporated in exosomes and could function freely in recipient cells after exosome-cell fusion. Using light-induced exosome targeting and release systems, this principle has been cleverly exploited for disease therapy.
Exosomes are heterogeneous, and the protein distribution is different due to the limited carrying capacity of exosomes, mechanical force, and randomness. The composition of individual exosomes is determined by the concentration of cargo in the vicinity of nascent exosomes. In addition, due to the differential expression of genes, exosomes secreted by antigen-presenting cells are rich in MHC II proteins and co-stimulatory proteins, while other types of cells lack the corresponding proteins. In addition, environment-induced gene expression may form exosome heterogeneity in different stages of diet, circadian rhythm, hormones, physical activity, infection, and cell cycle. Cytoplasmic and intramembrane protein concentrations also vary stochastically due to diffusion, dissociation, and other physicochemical processes of protein complexes, further amplifying the mechanistic drivers of differences in protein concentration at endosomes and at the plasma membrane. In addition, the change of exosomes can also be reflected by the space exclusion of cargoes. Larger exosome cargoes will occupy the space of new exosomes and affect the loading of other cargo proteins. In addition, there are also random fluctuations in gene transcription, leading to significant cell-to-cell differences in gene expression, thereby affecting the composition of exosomes. In summary, the heterogeneity of exosomes is not a problem that can or needs to be solved, but an inevitable result of the mechanism of exosome biogenesis.
g) Exosomal glycoconjugates and lipids
The outermost surface of exosomes is composed of attached surface proteins and a glycan canopy on some outer leaf lipids, and the exosomes derived from cancer cells are different, and the glycan characteristics increase with various glycosidases and sugar groups. expression of transferases. For example, some cancers express elevated levels of hyaluronan synthase 3, leading to the synthesis of long hyaluronan-like polymers that promote cancer growth and drive exosome biogenesis. Beneath the glycan canopy, the exosome membrane contains phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylinositols (PIs), phosphatidic acid (PA), cholesterol, Ceramides, sphingomyelin, and many low-abundance lipids. The total concentration of lipids in purified exosomes differs from plasma and other cell membranes, and these differences may provide clues to the mechanism of exosome biogenesis.
h) Exosomal RNAs
Exosomes contain RNA that can be transferred in a functional form to other cells and tissues to regulate gene expression in cells. Exosome-mediated miRNA transfer also extends the central dogma of molecular biology.
i) Exosomal DNA
Exosomes contain DNA, including single-stranded DNA, double-stranded DNA, genomic DNA, mitochondrial DNA, and even reverse-transcribed complementary DNA. Unlike other exosomal cargoes, it is unclear whether selective sorting of specific DNA occurs. Exosome-based DNA secretion may contribute to DNA quality control in the regulation of inflammation and may serve as a marker for cancer, viral infection, or chemotherapy resistance.
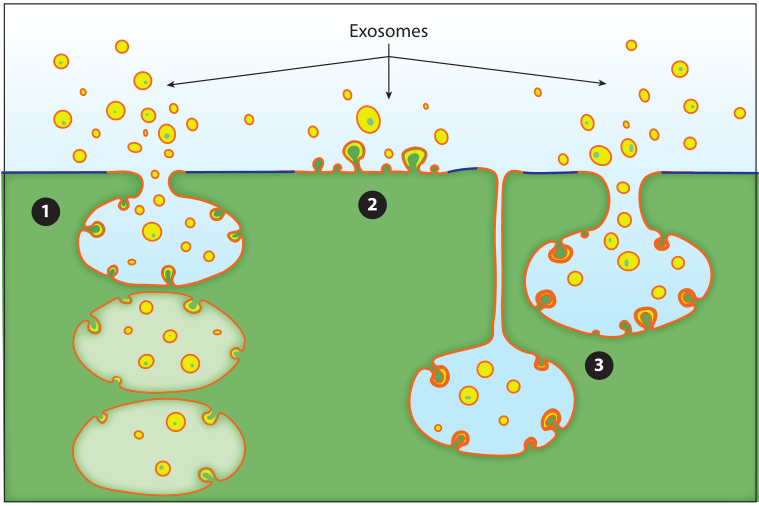
Figure 2: Three modes of budding of exosomes. Exosomes bud out into discrete endosomes through vesicles to release exosomes by fusion with the plasma membrane①, or directly from vesicle budding on the plasma membrane②, in addition, they can also connect compartments by shrinking the intracellular plasma membrane The junction of the exosomes delays the release of exosomes③.
In other organelle biogenesis pathways, proteins are recognized by short peptide signals and transported to cognate organelles, but in exosome protein sorting is mediated by plasma membrane anchors and oligomeric proteins in the cytoplasm Exosome secretion, such as: TfR aggregation and sorting, heat shock protein stress induction and exosome sorting. Oligomerization of proteins can induce budding of plasma membrane proteins, but there is currently no established model of how oligomerization and plasma membrane association generate biochemical signals for protein budding, nor is it clear how cells confine oligomers/aggregates size so that it does not exceed the physical carrying capacity of exosomes.
