Research on extracellular vesicles (EVs) and nanoparticles (NPs) is increasing rapidly, with recent years unveiling unprecedented complexity and diversity in vesicles. Consequently, our comprehension of cellular biology facilitated by the release of EVs and NPs, is in a perpetual state of evolution. Researchers from Robert J. Coffey’s group at Vanderbilt University Medical Center in the United States have published a comprehensive review delineating various types of EVs and NPs, accentuating recent research progress, and posing major unanswered questions. This pertinent content found its place in the preeminent international chemistry journal Trends in Cell Biology on February 1, titled “Extracellular vesicles and nanoparticles: emerging complexities.”
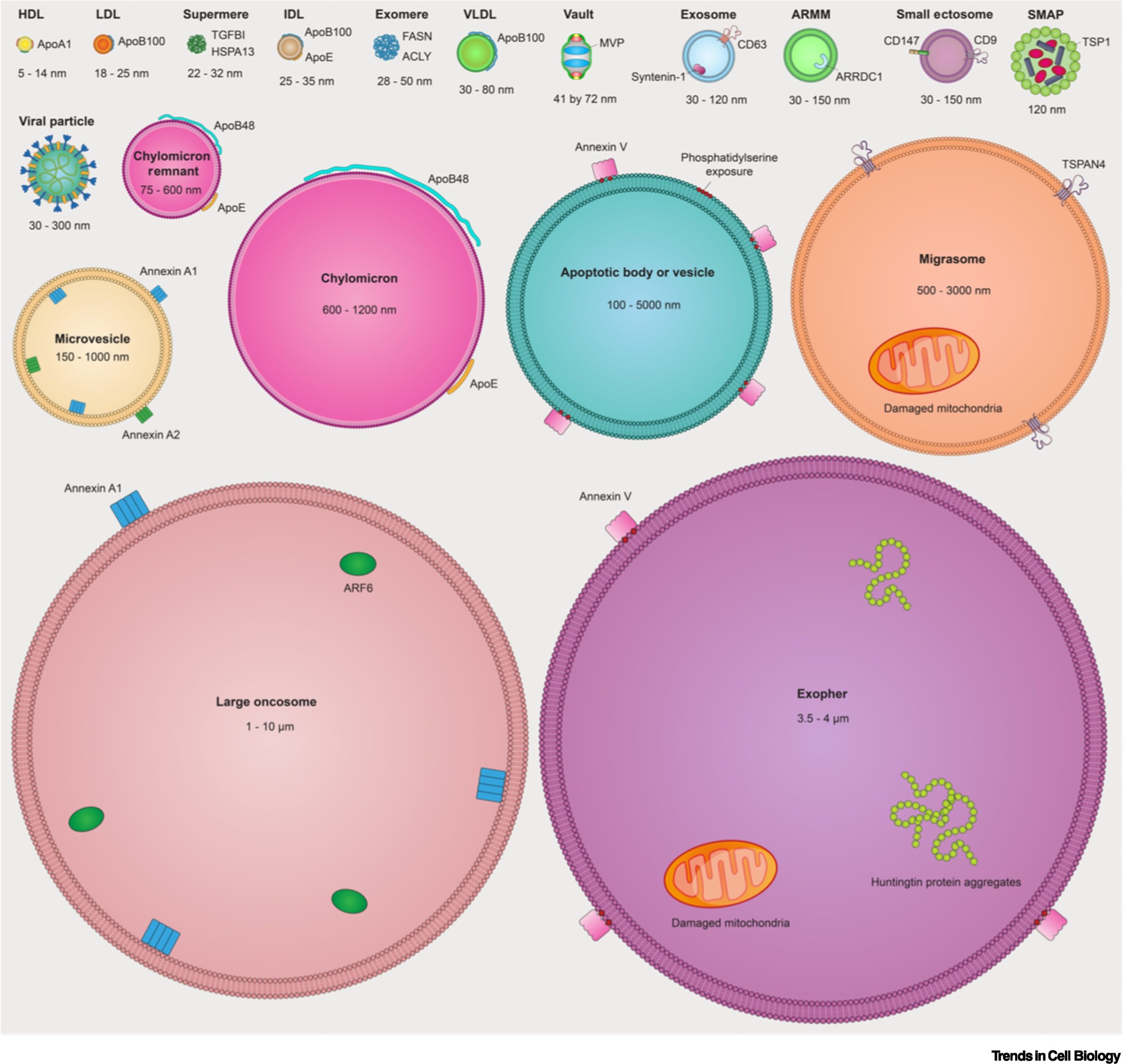
Cellular communication involves the secretion of signaling molecules, including proteins, lipids, and nucleic acids. These signaling molecules can be encapsulated in EVs, safeguarding them from rapid degradation and immune clearance, thereby facilitating local and long-distance communication between cells. EVs, lipid bilayer membrane-enclosed vesicles, are released by all cell types under normal and pathological conditions, discernible in various tissues and body fluids. The identification of diverse EV types is on the rise..
Recognition is growing that non-vesicular extracellular nanoparticles (NVEPs) without lipid bilayer membranes are present in substantial quantities in the extracellular space and body fluids. NVEPs include not only familiar entities such as lipoprotein particles, but also recently uncovered exomeres and supermeres. Technological advancements and heightened awareness of their intricacy and heterogeneity primarily underpin the discovery of EVs and NVEPs.
Initially perceived as a mechanism for normal cells toeliminate unwanted substances, or for cancer cells to promote tumor progression, EVs are now acknowledged to play multiple roles in intercellular communication. This involves the uptake of EV cargo followed by transfer to recipient cells or interaction between EV surface proteins and cellular receptors. Cell-cell communication mediated through EVs is crucial for maintaining normal physiological functions, and aberrant signaling by EVs is implicated in various disease states, including cancer, cardiovascular disease, neurological disorders, and immune diseases. EVs, along with their associated proteins, lipids, and nucleic acids (including miRNAs), present a rich source of potential biomarkers and therapeutic targets, with ongoing exploration of EVs as vehicles for drug delivery.
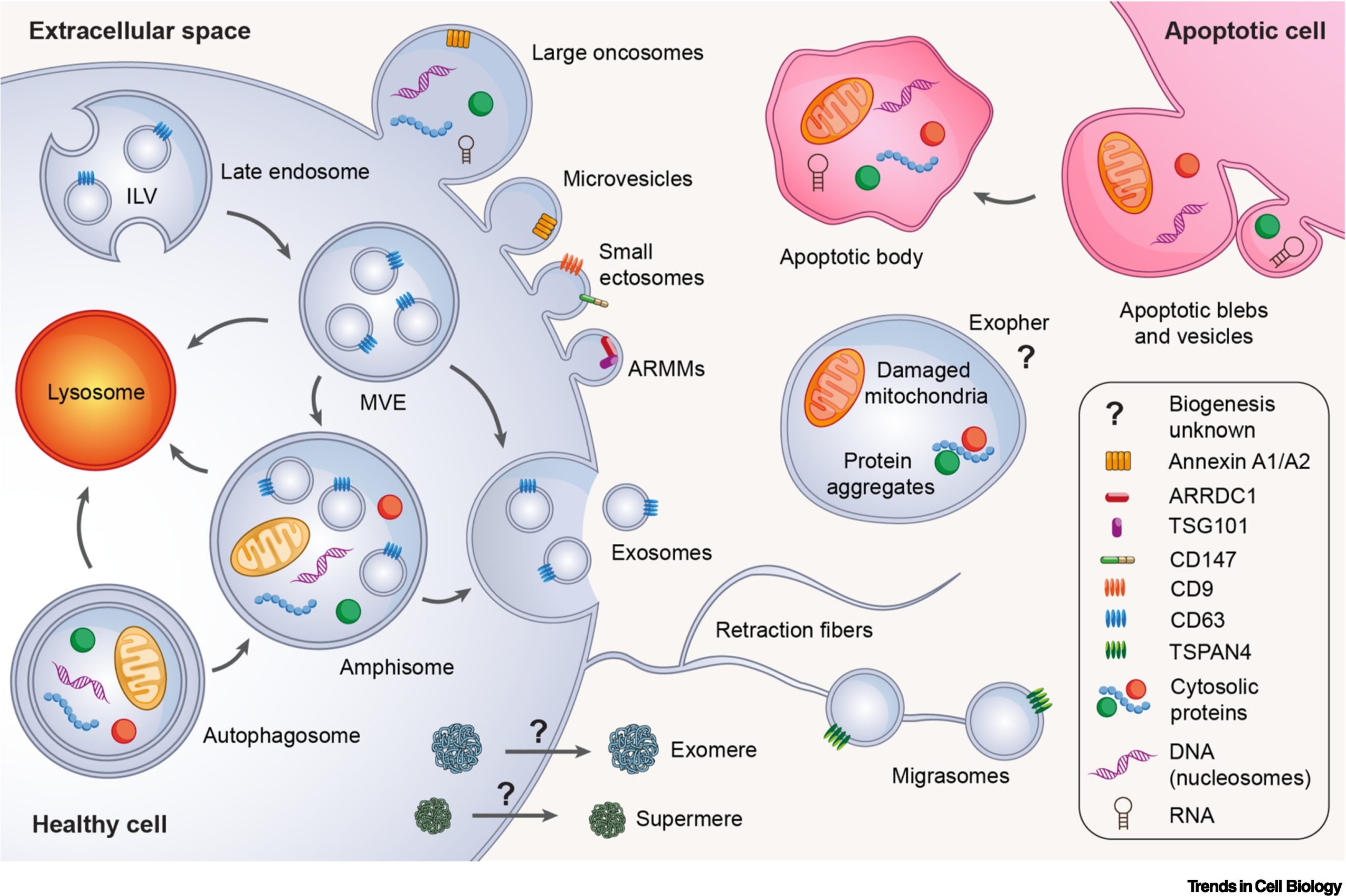
Classification of EVs is based on their origin and biogenesis, encompassing three major modes: apoptotic EVs (apoptotic bodies and vesicles) produced during apoptotic cell division, ectosomes produced by outward budding from the plasma membrane, and secretosomes produced by inward budding from endosomal compartments, later released by fusion with the plasma membrane. Given the plethora of EV types and uncertainty surrounding their biological origin, MISEV adopts a pragmatic approach, categorizing EVs as either “large EVs” (>200 nm) or “small EVs” (<200 nm). Thus, we review diverse EV types, NVEPs, and the latest advances concerning these extracellular particles.
The researchers posit that despite the surge in publications on EVs in recent years and the anticipated increase in papers on extracellular nanoparticles (NPs), more questions have surfaced. It is increasingly evident that cells employ abundant EVs and NVEPs for communication with neighboring and distant cells. What remains unclear, however, is the effective isolation, analysis and characterization of this intricate new medium of intercellular communication. Findings regarding EVs and NVEPs are contingent on the intricacies of experimental procedures, where slight variations in separation protocols can yield substantial changes in results and conclusions. Nevertheless, there is a general consensus that EVs represent a distinct class of vesicles. Regardless of size, many types of EVs are formed by direct budding from the plasma membrane, carrying numerous proteins previously believed to be primarily secreted in exosomes.
The researchers underscore that NVEPs, encompassing supermeres, exomeres, vaults, lipoproteins, and protein assemblies like ribosomes and ribonucleoprotein complexes, may account for the majority of extracellular RNA transport. The biogenesis of supermeres and exomeres remains unknown, and the mechanisms governing the packaging of RNA or DNA in EVs or NVEPs are largely unexplored. The extent of heterogeneity in NVEPs is unclear, and it is uncertain whether some components of supermeres and exomeres can be attributed to other distinct nanoparticles, such as ribosomes or ribonucleoprotein complexes. These are hotly debated topics that will undoubtedly be the focus of intense research in the upcoming years.
In conclusion, researchers anticipate that the diversity of EVs and other extracellular vectors carrying proteins, lipids, RNA, and DNA holds the potential to facilitate intercellular communication, contingent on the methods and techniques employed for isolation and characterization. This trend is expected to persist, as the technical development of EV and NVEP research remains a major focus in this relatively young yet burgeoning field. While extensive research has elucidated the role of EVs in cell-cell communication, the role of NVEPs in cell-to-cell communication remains relatively unexplored. A significant challenge lies in translating our understanding of the array of EVs and NVEPs possessed by a given cell into comprehension of how communication is facilitated within the intact organism.
Reference:
Jeppesen D K, Zhang Q, Franklin J L, et al. Extracellular vesicles and nanoparticles: Emerging complexities[J]. Trends in Cell Biology, 2023.
Related Services:
Nanoparticle Tracking Analysis-based Exosome Characterization
