Prostate cancer is one of the most malignant tumors in men and remains incurable due to its heterogeneous and progressive nature. Genetic and epigenetic changes play important roles in prostate cancer development. A collaborative team from multiple research institutions including Islamic Azad University in Iran published a review in the journal J Exp Clin Cancer Res, emphasizing the role of epigenetic changes in the development of prostate cancer. The review also discussed the expression levels of long non-coding RNAs (lncRNAs) and their interactions with other signaling networks involved in prostate cancer, with a particular attention to the application of exosomal lncRNA as biomarkers for prostate cancer.
The prostate is a walnut-sized organ located in the pelvic region at the tail end of the bladder. The incidence of prostate cancer is high in males, with 1 in 7 men diagnosed in the United States, and globally, 1 in every 25 men is diagnosed with prostate cancer. Prostate enlargement that occurs with age is termed benign prostatic hyperplasia (BPH) and is associated with some symptoms observed in men over 60 years old, such as polyuria. Due to the histopathological and molecular similarities, BPH is considered a stage in the initiation of prostate tumor. However, the exact underlying mechanisms by which BPH leads to prostate tumor development are not fully understood. Prostate-specific antigen (PSA) testing is used for diagnosis, thus contributing to the higher incidence of prostate cancer in developed countries.
Prostate cancer is one of the malignant tumors in men. Newly released statistics show that compared with 2020, the incidence of prostate tumors has increased, with 248,530 new cases and 34,130 deaths. Due to advances in the medical field in recent years, especially in developed countries, the survival and prognosis of patients with prostate tumors have improved significantly. This can be observed in the 5-year survival rate of prostate tumor patients, which was 97.8% in 2016, significantly better compared to 66.9% in 1975. Age, race, heredity, family history, obesity, and smoking are the most common risk factors for the development of prostate tumors. If treatment for prostate cancer fails, a new form called castration-resistant prostate cancer (CRPC) develops, which is a problem in the clinical course. Some of the major genes mutated in CRPC prostate cancer include androgen receptor (AR), TP53, RB1, PTEN, and DNA damage repair (DDR).
There are many ways to treat prostate cancer. Surgery is beneficial in the initial stages of prostate cancer. For advanced and metastatic prostate cancer, chemotherapy and its combination with radiation therapy are used. In addition, androgen deprivation therapy (ADT) is widely used in the treatment of prostate cancer cells due to their dependence on androgens. Immunotherapy, including the use of immune checkpoint inhibitors, antibody-mediated radioimmunotherapy, antibody-drug conjugates, and bispecific antibodies, is a promising option in prostate cancer treatment. However, due to the aggressive nature of prostate cancer cells, they become resistant to different treatments and can activate pro-tumor signaling pathways to induce chemoresistance, radioresistance, ADT resistance, and immune resistance. Therefore, strategies should be sought to reverse treatment resistance in prostate tumors, and this goal is achieved through pharmacological and genetic interventions. Wnt, STAT3, Hedgehog (Hh), PTEN, PI3K/Akt, NF-κB, and SPOP are signaling networks that undergo aberrant expression in prostate cancer. Notably, noncoding RNAs (ncRNAs) have received special attention in prostate cancer because ncRNAs have dual roles in increasing or inhibiting tumor progression.
In this review, researchers describe in detail the functions of lncRNAs in prostate tumors. The study first introduces long non-coding RNAs (lncRNAs) and their biogenesis and biology, as well as their pathological functions. The researchers then specifically discuss the role of lncRNAs in the progression rate (growth and migration), chemoresistance, and radioresistance of prostate tumor cells. Furthermore, the role of lncRNAs as upstream mediators in regulating major molecular pathways in prostate cancer is also discussed. Finally, current applied therapies targeting lncRNAs in prostate cancer treatment are described.
The researchers believe that aberrant expression of lncRNAs in prostate cancer is well documented and that the rate of tumor cell progression is regulated by affecting molecular pathways such as STAT3, NF-κB, Wnt, PI3K/Akt, and PTEN. Furthermore, lncRNAs regulate the radiotherapy resistance and chemoresistance characteristics of prostate tumor cells. Overexpression of tumor-promoting lncRNAs such as HOXD-AS1 and CCAT1 can lead to drug resistance. In addition, lncRNAs can induce immune evasion in prostate cancer by upregulating PD-1. Pharmacological compounds such as quercetin and curcumin have been used to target lncRNAs. Furthermore, siRNA tools can reduce the expression of lncRNAs, thereby inhibiting prostate cancer progression. The prognosis and diagnosis of prostate tumors during the clinical course can be assessed by lncRNAs. Importantly, studying the expression levels of exosomal lncRNAs, such as lncRNA-p21, in the serum of prostate cancer patients can serve as reliable biomarkers.
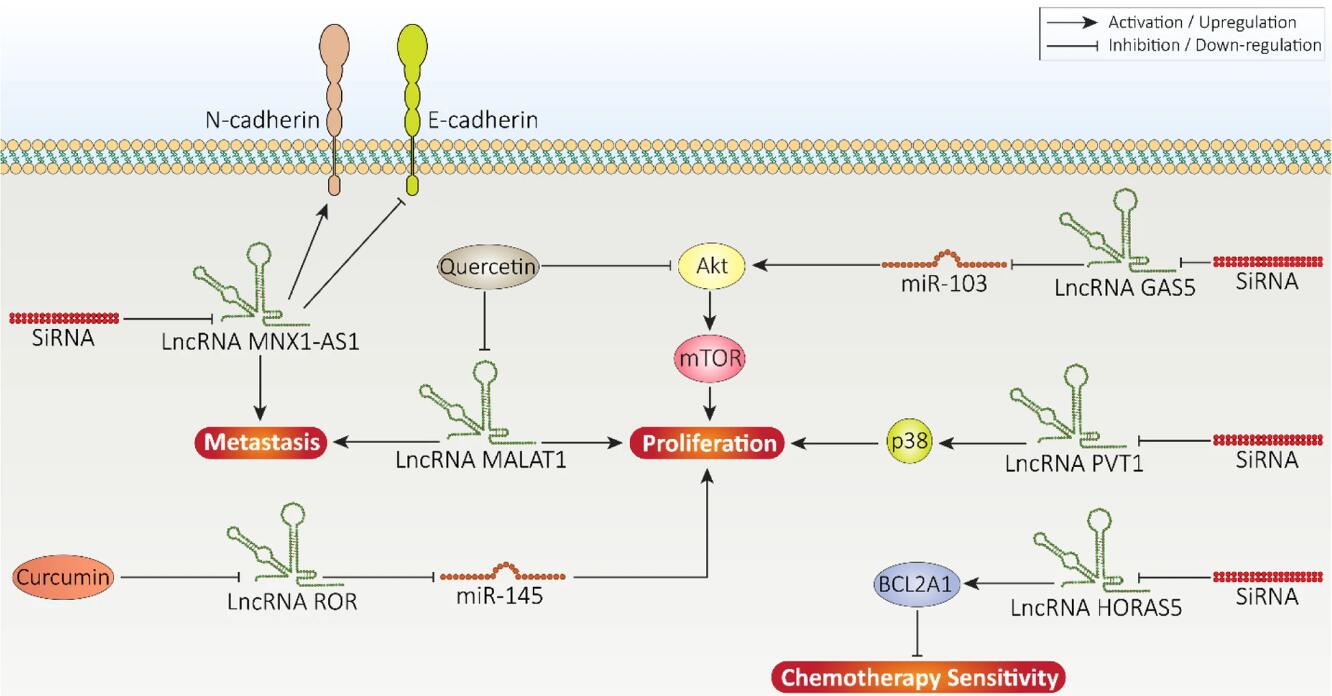
Prostate Cancer Therapy Targeting lncRNA
Exosomal lncRNA
In recent years, special attention has been paid to extracellular vesicles (EVs) obtained from cancer and non-cancer cells. EVs are mainly divided into three categories, including exosomes, microvesicles, and apoptotic bodies, which play functional roles under physiological and pathological conditions. As nano-extracellular vesicles, exosomes exist in the TME, and various body fluids such as blood, saliva, pancreatic duct fluid, and amniotic fluid can participate in their transport to distant tissues and organs. Additionally, they also function through both autocrine and paracrine fluids. Exosomes provide communication between various cells, and they contain various macromolecules such as proteins, lipids, and, most importantly, nucleic acids. Exosomes originate from endosomal processing and are reported to contain ncRNAs, especially lncRNAs. Therefore, it is crucial to reveal the role of exosomal lncRNA in cancer.
It is worth mentioning that exosomal lncRNA can be used to distinguish prostate cancer from BPH. Therefore, by developing novel imaging methods for tracking exosomes, such as Antares2-mediated bioluminescence resonance energy transfer (BRET), a revolution in cancer diagnosis can be launched.
LncRNA is a potent regulator of different molecular pathways in prostate cancer, and microRNA (miRNA) is one of the most common downstream targets of lncRNA. An experiment showed that certain lncRNAs are enriched in prostate cancer exosomes, and lncRNAs that regulate miRNA expression are one of them. The exosomal lncRNAs ELAVL1 and RBMX are enriched in prostate cancer because of their ability to regulate the expression levels of miRNAs such as miRNA-17, miRNA-18a, miRNA-20a, miRNA-93, and miRNA-106b. Exosomes can accelerate the transfer of lncRNA to the extracellular environment, and based on the role of lncRNA as a tumor suppressor or tumorpromoting factor, it will affect the proliferation and invasion of prostate cancer cells. Although some studies have evaluated the role of exosomal lncRNA in prostate cancer, it seems that these types of lncRNA can be considered novel diagnostic and prognostic factors for prostate cancer, and their expression levels are important for distinguishing BPH and prostate cancer. Furthermore, more diagnostic tools should be developed to detect exosomes in prostate cancer.
Table. An Overview of lncRNAs Involved in Prostate Cancer Progression/Inhibition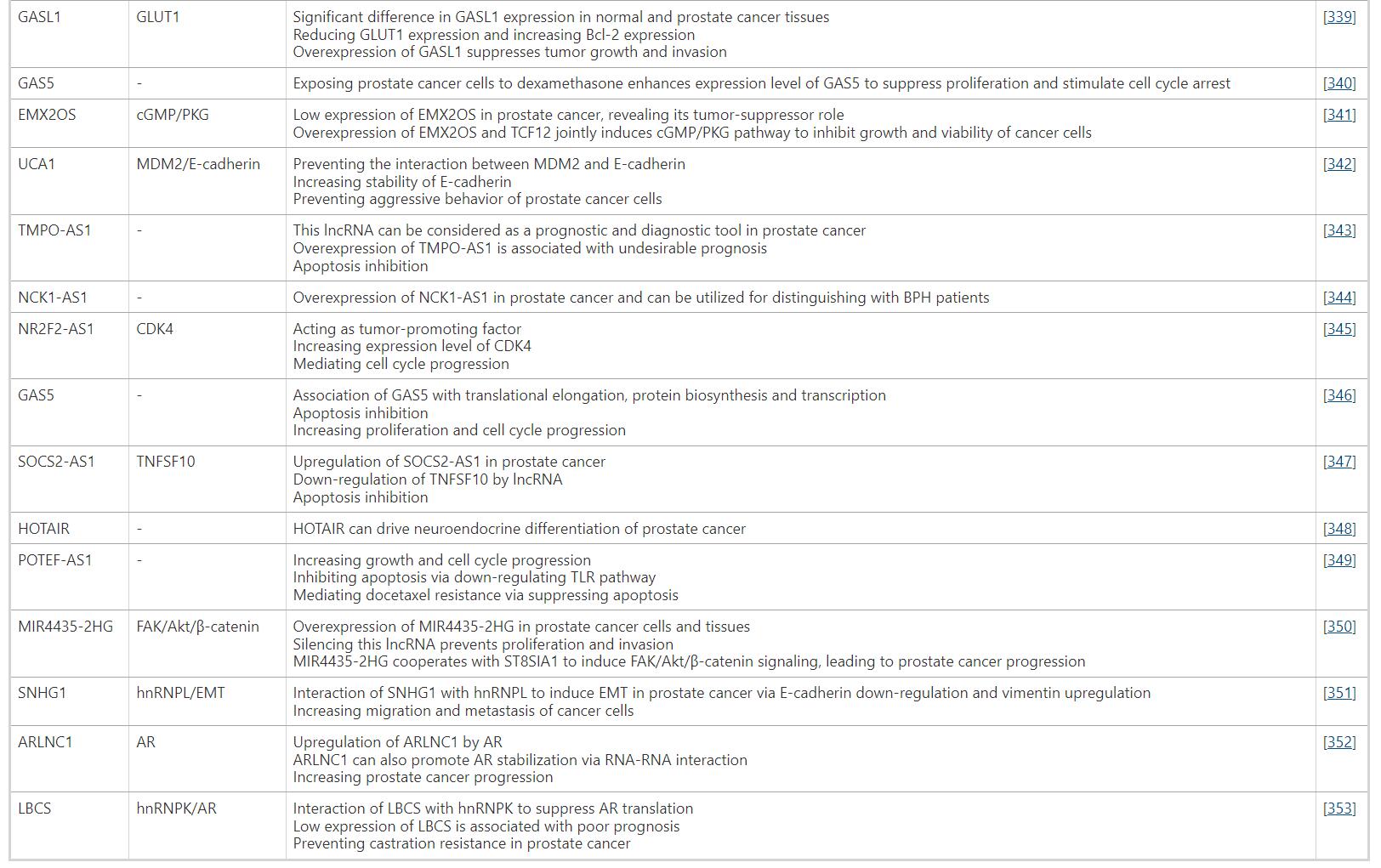
Reference:
Mirzaei S, Paskeh MDA, Okina E, et al. Molecular Landscape of LncRNAs in Prostate Cancer: A focus on pathways and therapeutic targets for intervention. J Exp Clin Cancer Res. 2022;41(1):214. Published 2022 Jul 1. doi:10.1186/s13046-022-02406-1
Related Services:
