Extracellular vesicles (EVs), released from various cell types under physiological and pathological conditions, are carriers of cell-to-cell communication. Recently, the Journal of Controlled Release published a review paper entitled ” Promotion or inhibition of extracellular vesicle release: Emerging therapeutic opportunities“.

This article reviews the biological mechanism of EVs and exosomes, the methods of regulating EVs release, and the progress of treatment, and discusses the side effects and current challenges of EVs therapeutics .
Extracellular vesicles (EVs) are released from cells under pathophysiological conditions and can be isolated from various body fluids. According to their biogenesis mechanism, EVs are divided into three types: exosomes (30-100nm EVs originate from the endocytic pathway, microvesicles (MVs) (50-1000nm EVs sprouting from the plasma membrane), and apoptotic bodies (1-5-μm EVs from apoptotic cells). Current research focuses on the first two types of vesicles and collectively refers to them as EVs.
As the carrier of cell-to-cell communication, EVs have a “double-edged sword” function in regulating the physiological and pathological processes of the human body. Under physiological conditions, EVs mediate the communication between cells and tissues and participate in the maintenance of homeostasis, for which EVs have been used as a recommended biological treatment method in the fields of cell-free regeneration, anti-cancer vaccines, and spatiotemporal delivery of drugs (Figure 1). The secretion and release of extracellular vesicles are related to a variety of physiological and pathological conditions, and the control and intervention of their release have important significance and application value.
There are several examples that illustrate the role of EVs in treating diseases. (1) To a large extent, EVs derived from mesenchymal stem cells have the tissue regeneration and damage repair effects possessed by mesenchymal stem cells. (2) The tumor peptide activates EVs derived from dendritic cells to induce a strong anti-tumor immune response, which can be used as a cell-free anti-cancer vaccine. (3) EVs are ideal drug delivery carriers because they are easy to circulate, can avoid immune rejection, and reduce certain risks, such as uncontrolled cell division and cancer-causing contamination. (4) Neurons in the brain communicate by releasing EVs to promote local and distal synaptic plasticity. However, current production methods have low EVs yields, while clinical disease treatment requires a large amount of EVs. For example, in order to achieve biological results, mice usually require a dose of 109-1011 EVs per treatment, which may require several liters of conditioned medium and months of collection. Insufficient EVs production greatly hinders its clinical application. Therefore, in order to overcome this bottleneck in the implementation of cell-free therapy, it is important to improve the production and quality of EVs.
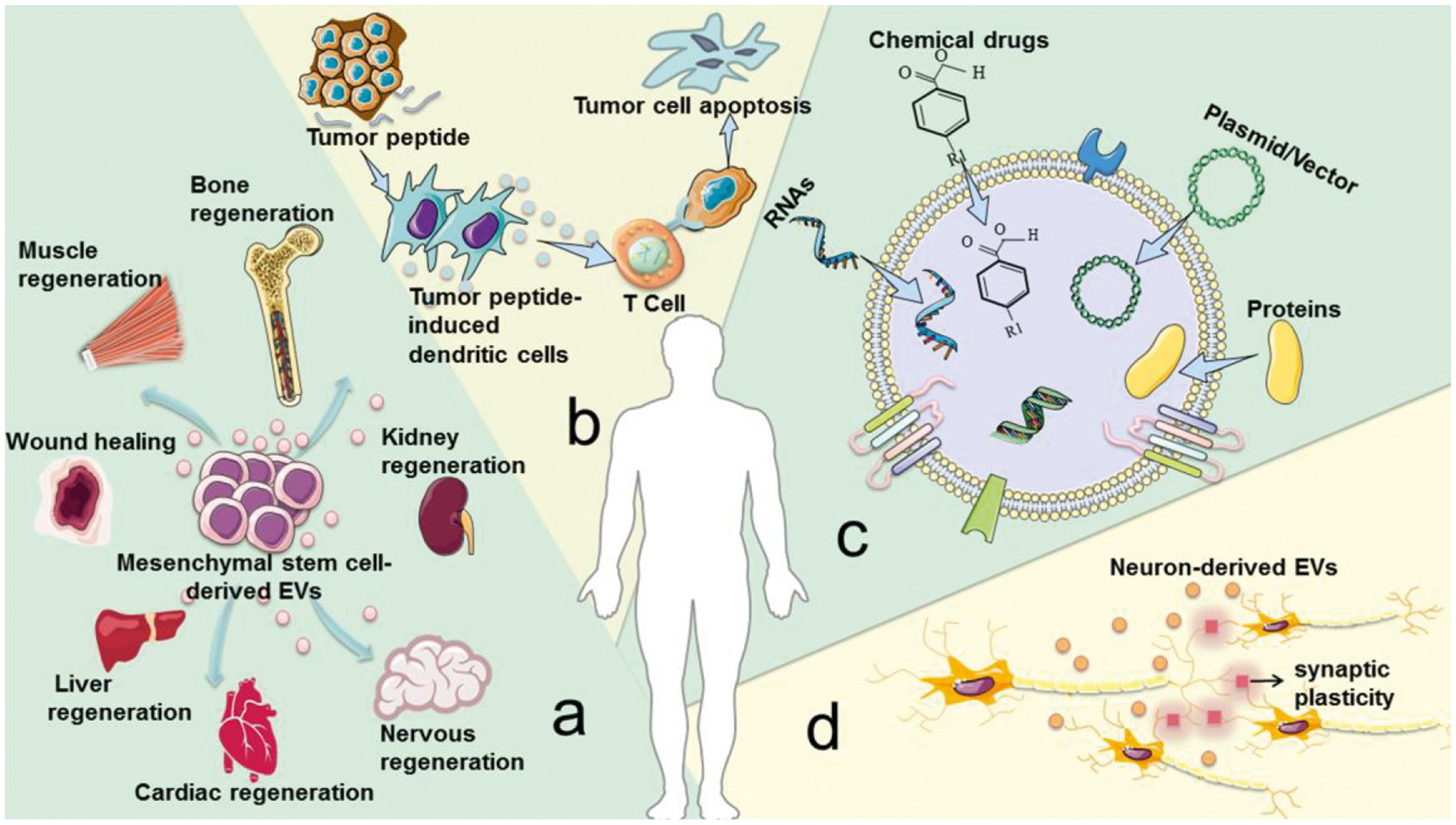
Fig.1 Advantages of EVs under physiological conditions
In addition to being used as a treatment method, EVs are also considered to be accelerating factors in the progression of many diseases (Figure 2). (1) EVs derived from tumors induce immunosuppression, tumor angiogenesis, chemotherapy resistance, and matrix remodeling, promoting tumor development and metastasis. (2) EVs secreted by the airway are involved in the pathogenesis of allergic airway inflammation under allergen exposure. (3) Smooth muscle cells in cardiovascular tissues release calcified EVs. These EVs contain cargo that promotes calcification under the pathological conditions of atherosclerosis. Once these EVs are released into the extracellular matrix, they will have a serious risk of aggregating and forming plaques. (4) During sepsis, macrophages stimulated by lipopolysaccharide will release more EVs containing pro-inflammatory cytokines, and this increase of EVs is related to the progression of myocardial dysfunction. Many similar studies on the role of EVs in cancer, trauma, autoimmune diseases, infectious diseases, and cardiovascular diseases have been published. Therefore, inhibiting the release of EVs to reduce the negative effects of diseases has become a new treatment strategy.
EVs under pathological conditions can significantly promote immune escape and tumor metastasis, viral particle transmission, or the development of atherosclerosis. Inhibiting the secretion of the above-mentioned EVs to delay the progression of these diseases is becoming a new treatment strategy. Therefore, the promotion and inhibition of EVs release have great research potential and broad application prospects. In this review, the authors use the biogenesis mechanism of different EVs as the overall framework, and respectively elaborated on the methods of regulating the release of exosomes and microvesicles. The therapeutic effects of EVs release regulation in tumors, injuries, infections, vaccine development, regenerative medicine, immune diseases, neurodegenerative diseases, etc. are summarized, and future prospects and challenges in this field are discussed, providing ideas for the therapeutic application of EVs.
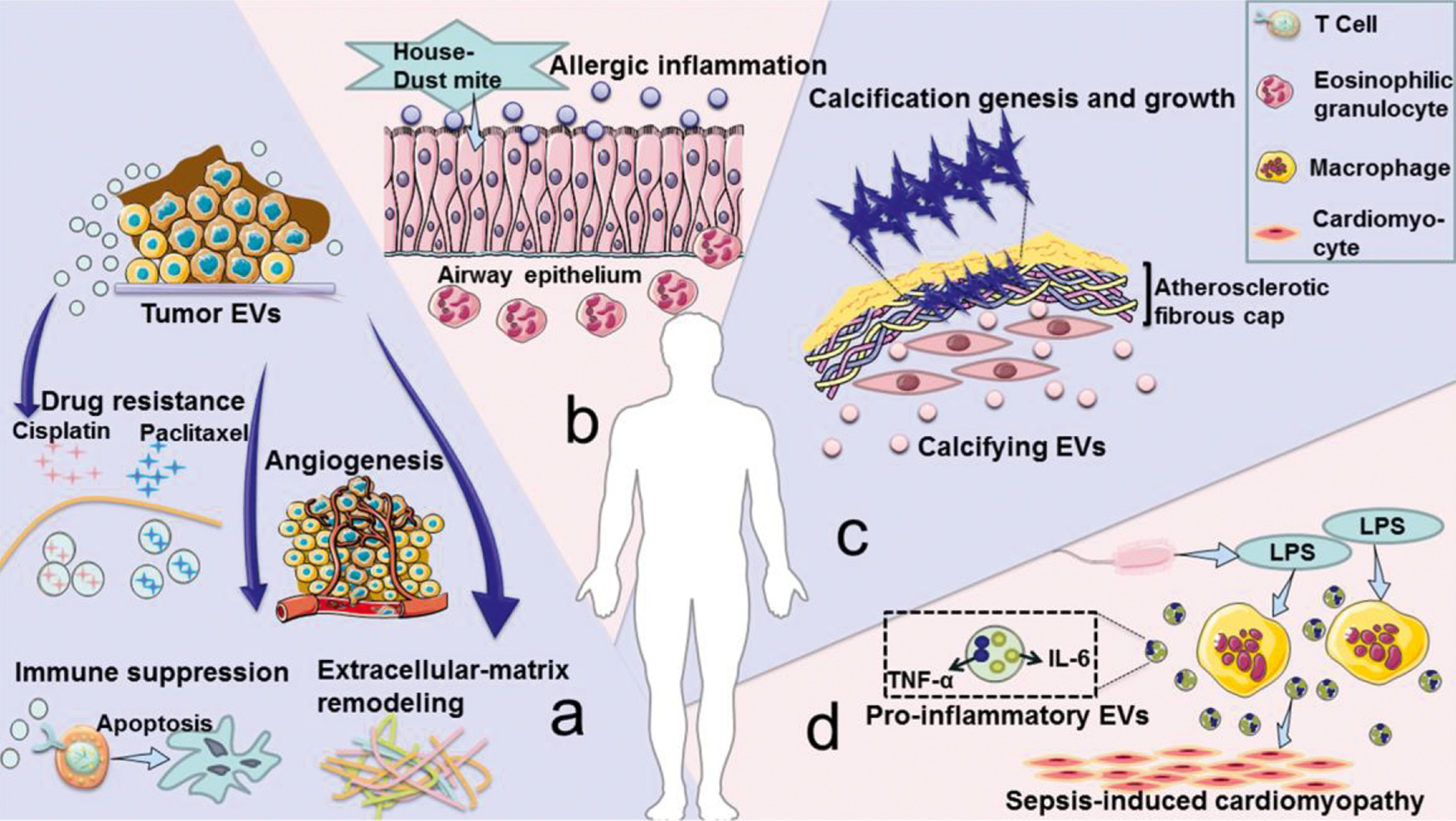
Fig.2 Disadvantages of EVs under pathological conditions
In the chapters on exosomes release mechanism and derived regulation methods and therapeutic potential, the author systematically analyzed three key stages of exosomes release (Figure 3), including the early endosome maturation into multivesicular bodies, the prevention of multivesicular bodies from being degraded by lysosomes, and the transport and fusion of multivesicular bodies to the plasma membrane. Further, this part discussed the use of hand mycin, GW4869, apyrimod, monensin, Suxiao Jiuxin Pill, dimethyl amiloride, and other drugs to regulate the above key stages, as well as the treatment effect of diabetes, kidney injury, ovarian cancer, melanoma cancer, lung cancer, allergic asthma, myocardial ischemia, septic cardiomyopathy, cardiac hypertrophy, and other diseases.
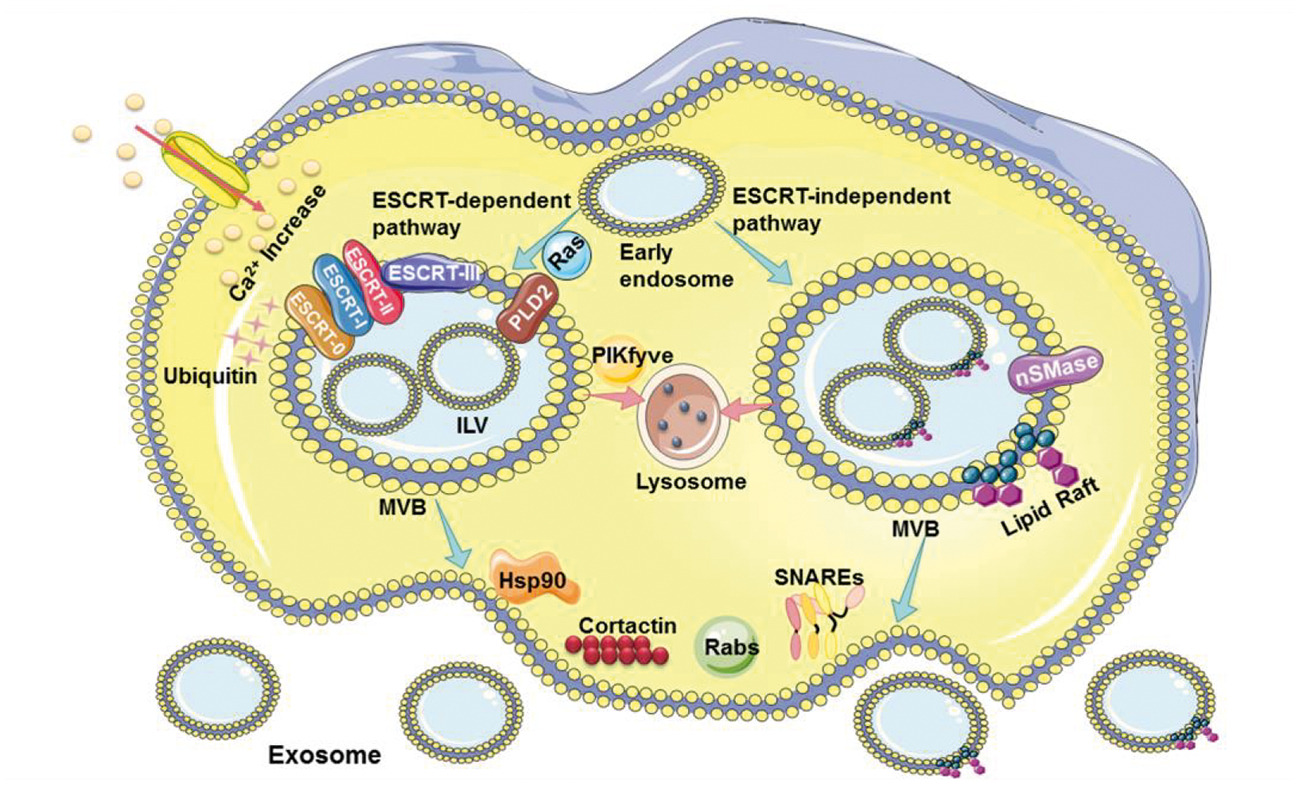
Fig.3 Schematic of exosome biogenesis
In addition, the author analyzed the controllable steps in the release of the two microvesicles, the lipid reorganization of the cell membrane and the reorganization of the actin cytoskeleton (Figure 4). The article also discussed the use of pantethine, calpain inhibitor, U0126, Y27632, Chloramidine, and other drugs to regulate the release of microvesicles, and their treatment progress of systemic sclerosis, cerebral malaria, atherosclerosis, pancreatic cancer, prostate cancer, and breast cancer, and other diseases.
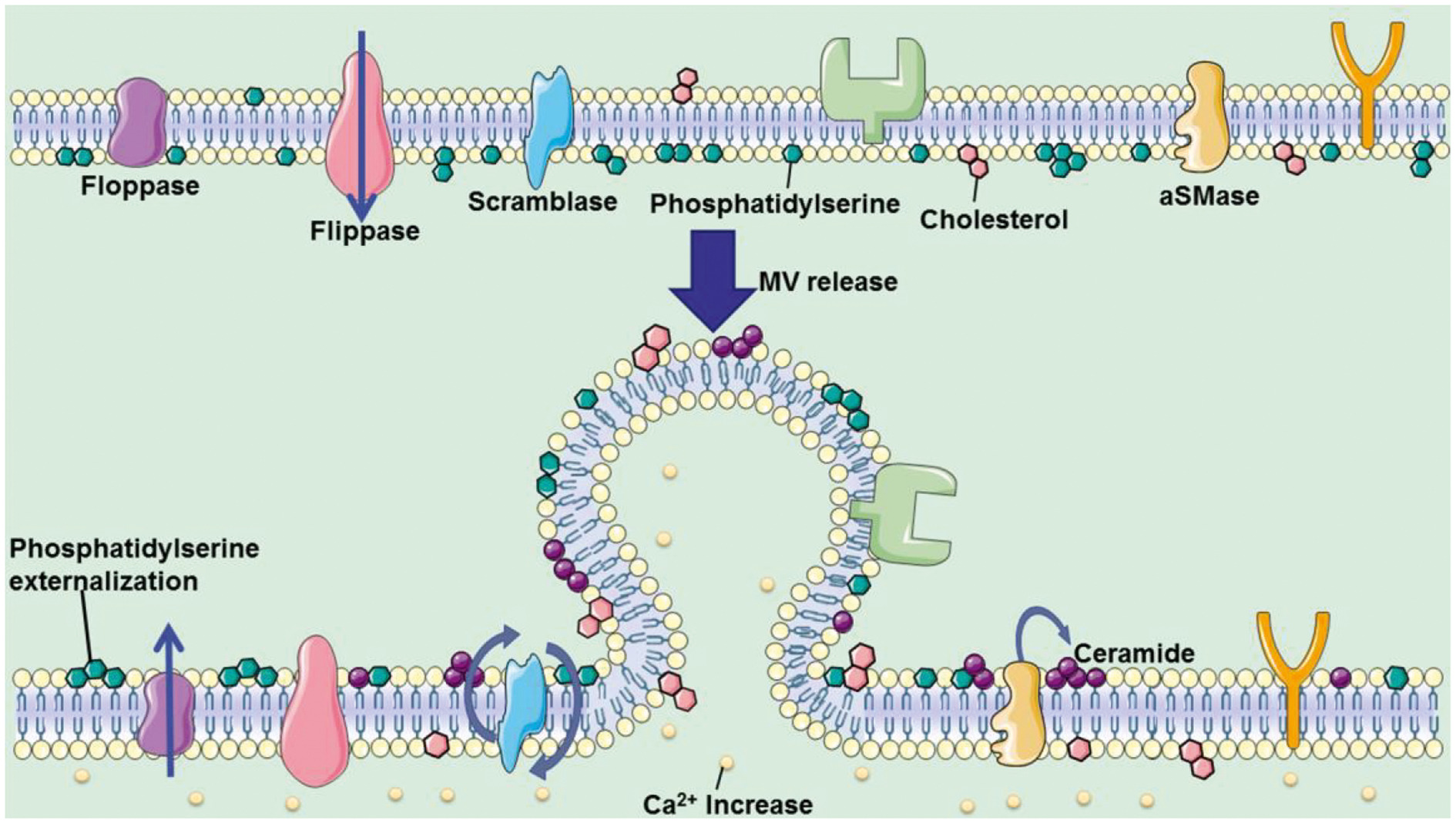
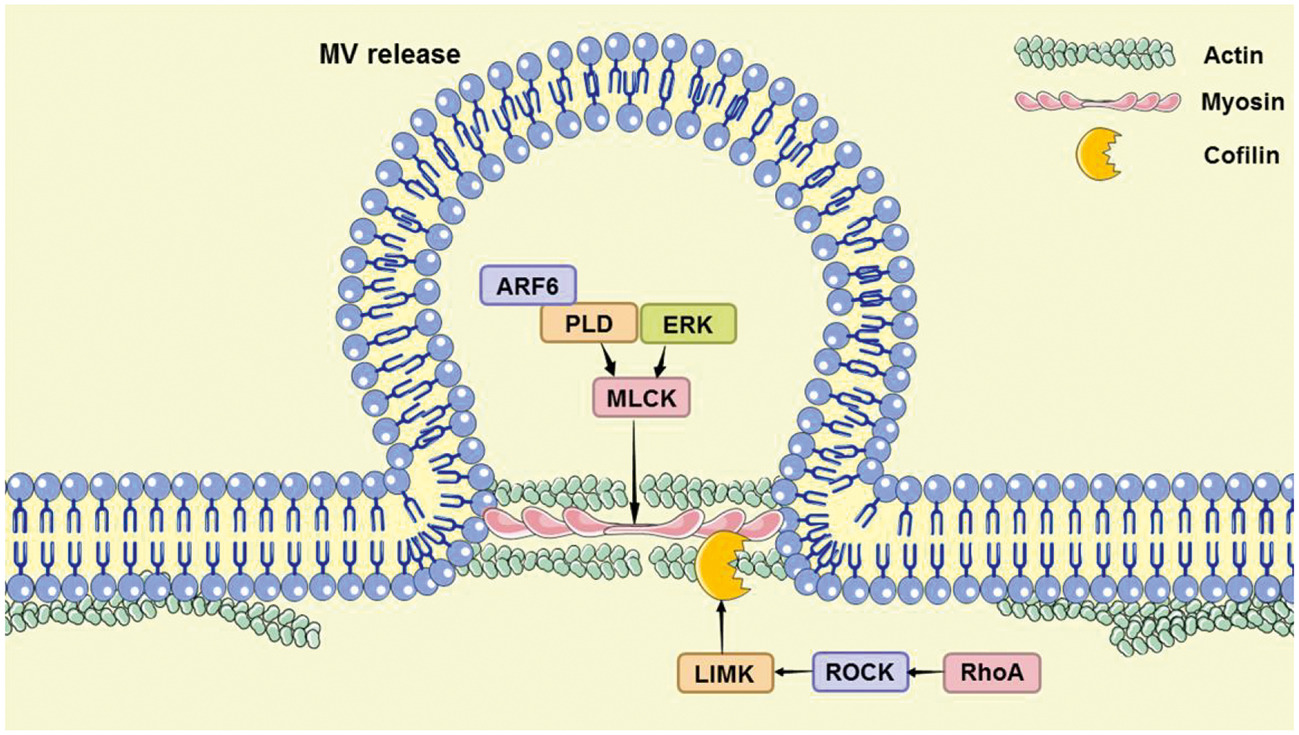
Fig. 4. Two pathways of microvesicle release regulation
In addition to the above known categorized mechanisms, the author further explored other conditions that cannot be summarized in the above-mentioned EVs biogenesis mechanism but have been reported to affect the release of EVs, such as certain stress, damage, current, temperature changes, and the homologous interactions between specific cells, etc. The author analyzed the progress made in the production of anti-cancer vaccines and engineered EVs by using the above methods to regulate the release of EVs.
At present, the therapeutic field of EVs is still in its infancy, and its characteristics and biological mechanism are not yet fully understood. Basic research and clinical trials on regulating the release of EVs are also expanding and developing. Finally, the author of this article discussed the possible side effects of regulating the release of EVs, as well as some challenges before clinical applications, such as (1) The need for determining the recognized efficacy test system, safety test system, and EVs separation and purification method. (2) The necessity of improving the targeted therapy ability of EVs, (3)Limitations of the existing EVs tracing technology in monitoring their biodistribution and release effect in the body; (4) How to minimize the side effects and drug toxicity induced by the control of EVs release, (5) Whether regulating the release of EVs will cause changes in EV content or function. Each breakthrough of these challenges will accelerate the clinical transformation of this emerging field. In the future, enhancing the release of EVs to achieve the large-scale production of engineered EVs, achieving efficient delivery of nucleic acids and other drugs to different target receptors, or directly inhibiting the release of EVs to reduce their harmful effects in related diseases, will bring new treatments and opportunities in clinical applications.
Reference:
Promotion or inhibition of extracellular vesicle release: Emerging therapeutic opportunities. J Control Release. 2021, 10:136-148
