In a recent study, researchers from the University of Heidelberg, Mannheim Medical School, and Newcastle University uncovered a novel mechanism of immune cell trafficking through the investigation of vascular disease. This knowledge may open up new therapeutic approaches for a large number of inflammatory diseases. The remarkable findings of this study were recently published online on April 25, 2023, in the journal Immunity under the title “The RNA editor ADAR2 promotes immune cell trafficking by enhancing endothelial responses to interleukin-6 during sterile inflammation.”
When our body is injured or infected by a pathogen, chemical signals called cytokines allow immune cells to leave the bloodstream and travel through the walls of blood vessels to the site of injury or infection. This process of immune cell transit is essential to the body’s natural defenses.
However, if this process is activated over time, it can lead to chronic tissue inflammation and organ damage, which, in turn, promotes the development of chronic inflammatory diseases including heart disease and cancer. Thus, immune cell trafficking is an important parameter that can be used to regulate the inflammatory process and is a focus of drug development for the treatment of a wide range of diseases.
These authors investigated ischemic diseases as an example. During ischemic diseases, our body experiences hypoxia in the affected tissues, triggering the production of cytokines. The most common consequence of chronic ischemic heart disease is heart failure.
Professor Konstantinos Stellos, co-corresponding author of the paper, said, “Chronic inflammation is an important mechanism in ischemic heart disease. People who have had a heart attack are at high risk of having a second heart attack, developing heart failure, or experiencing other complications. If we understand how immune cells are recruited to the heart after an acute heart attack or in chronic ischemic heart disease, we can develop new treatments to reduce this risk, known as residual risk.”
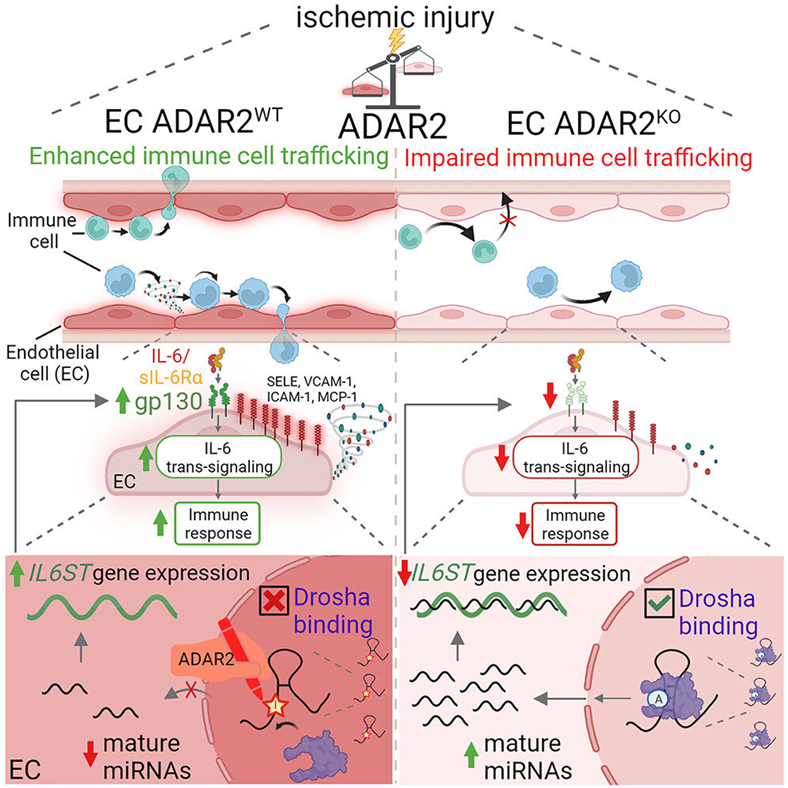
Their discovery that an RNA enzyme called ADAR2 (Adenosine Deaminase Acting on RNA-2) orchestrates the inflammatory response in the heart or other ischemic muscle tissue makes it a promising therapeutic target for a variety of inflammatory diseases. ADAR2 catalyzes a base in RNA at a specific location in RNA, the adenosine, to another base, the inosine, a process known as RNA editing.
These authors determined the exact mechanism by which RNA editing controls immune cell trafficking in ischemic diseases, from single nucleotide resolution to the organ level.
Professor Stellos and Dr. Aikaterini Gatsiou, both co-corresponding authors and the first author of the paper, provide insight by stating, “We firmly believe that a major hurdle in expediting the development of novel therapies lies in the tendency of basic research to rely solely on singular levels of analysis for scientific observations. As complex organisms, we require a multifaceted approach to uncovering new pathways that can serve as potential targets for therapeutic interventions. The most effective methodology involves the utilization of human and murine primary cells, experimental disease models, and human biopsies, enabling exploration and testing across all levels of the mechanism. This comprehensive approach was precisely our intention when designing this groundbreaking study. Notably, this study was made possible through the invaluable contributions of several experts hailing from diverse research domains.”
Not only may ADAR2 be a therapeutic target in its own right, but its activity may be used as an alternative tool to gene editing techniques. DNA editing using CRISPR/Cas9 gene scissors now allows scientists to specifically modify an organism’s DNA to correct disease. However, the technique also hides risks, as it leads to irreversible changes in DNA.
According to Dr. Gatsiou, “Utilizing RNA editing offers a more promising approach to developing treatments for inflammatory diseases like heart failure, cancer, and autoimmune diseases. RNA editing operates independently of DNA, targeting specific areas with reversible and dose-controlled effects due to RNA’s short lifespan. Our study presents a comprehensive framework for advancing such initiatives.” Professor Stellos added, “It is intriguing that various research groups are exploring the utilization of human ADAR enzymes as manageable RNA editing tools.”
Given that inflammation serves as a common mechanism in several diseases, the findings of this new study have the potential to instigate therapeutic advancements in multiple conditions.
Reference
1. Gatsiou, Aikaterini, et al. “The RNA editor ADAR2 promotes immune cell trafficking by enhancing endothelial responses to interleukin-6 during sterile inflammation.” Immunity 56.5 (2023): 979-997.
