Recombinant Adeno-Associated Virus
Creative Biolabs manifests as a world-leading expert in recombinant adeno-associated virus (rAAV) development and provides our clients with reliable products in order to meet their various research needs. Taking advantage of the well-established recombinant virus platform, various AAV serotypes and engineered strategies are available for clients to support their projects. We will apply rigorous criteria that have been established for rAAV production to assure their quality for every gene therapy study. We also provide advanced adeno-associated virus vector construction services from design, purification, titration, to toxicity and safety determination.
Our Products
To date, the rAAV is mainly used in gene therapy to deliver DNA to target cells and has been shown to be safe and effective in preclinical and clinical settings. As a non-pathogenic and actively investigated gene therapy vehicles, rAAV vectors have proven to be highly efficient in gene delivery to a wide variety of cell types, even in the body. A number of AAV serotype products are available at Creative Biolabs, including AAV2, AAV5, AAV8, and AAV9 serotype vectors with distinct tissue-tropism, and long-term transgene expression. Through rationally design and optimization, various AAV serotype vectors are capable of targeting specific tissues and organs for gene therapy, which further expands their therapeutic landscape.
Recombinant Adeno-Associated Virus (rAAV) Introduction
Recombinant adeno-associated virus (rAAV) is a transformative gene delivery platform derived from a naturally occurring, non-pathogenic parvovirus. Wild-type AAV is a dependent virus, requiring co-infection with a helper virus (such as adenovirus or herpesvirus) for efficient replication. This inherent deficiency constitutes an important safety feature, as rAAV vectors cannot replicate autonomously in target cells. In the engineering of rAAV vectors for gene therapy applications, all natural viral genes (rep and cap) in the viral genome are strategically removed, retaining only the essential inverted terminal repeat sequences (ITRs). These ITR sequences flank the therapeutic transgene and are critical cis-acting elements necessary for genome replication and packaging.
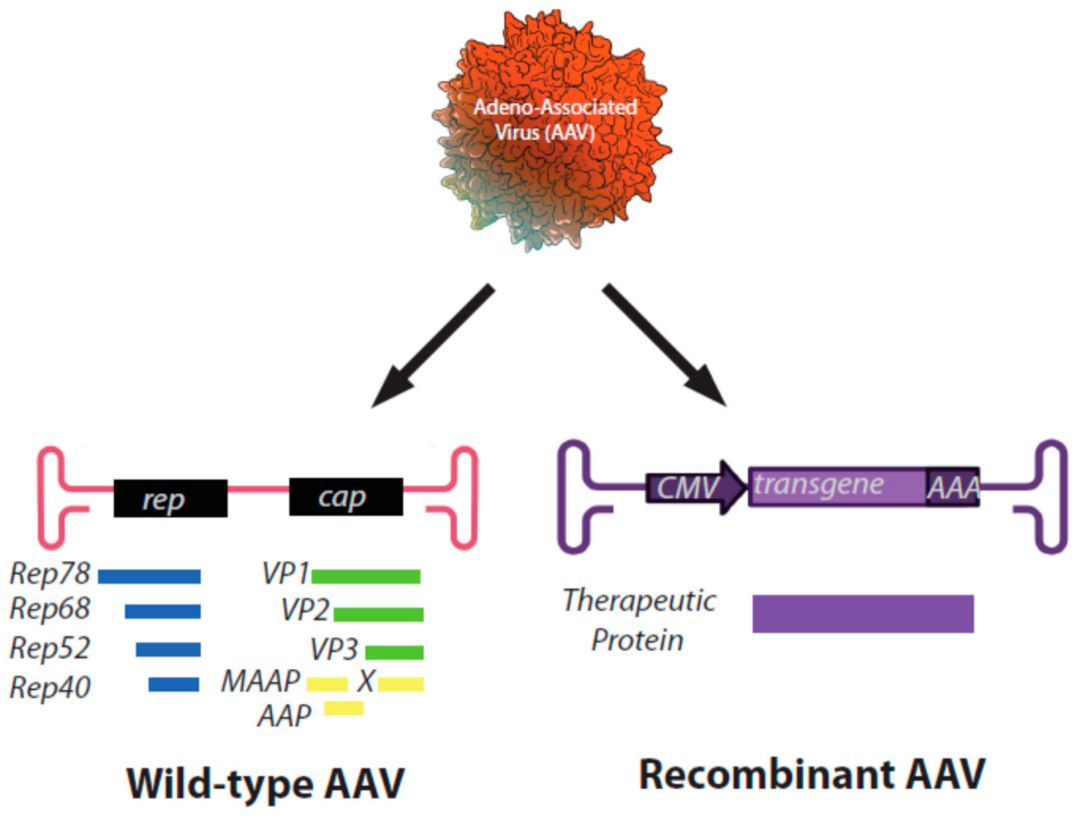 Figure 1. Schematic representation of genomes of a wild-type AAV virus (left) and a recombinant AAV particle (right). Proteins encoded by each ORF are listed below the appropriate gene.1
Figure 1. Schematic representation of genomes of a wild-type AAV virus (left) and a recombinant AAV particle (right). Proteins encoded by each ORF are listed below the appropriate gene.1
Types of Recombinant Adeno-Associated Virus
The wide diversity of AAV viral vectors provides researchers with a versatile toolbox for targeted gene delivery. This diversity stems from naturally occurring serotypes and engineered capsids, each possessing unique tissue tropism and transduction efficiency.
Naturally occurring AAV serotypes, primarily including AAV1 to AAV12, exhibit different cellular tropisms based on the interaction of their capsid proteins with specific cell surface receptors. For example:
- AAV1: Shows high transduction efficiency in muscle and nerve tissue
- AAV2: Is one of the most widely studied serotypes, suitable for nervous system applications
- AAV5: Shows a preference for lung, eye, and nerve tissue through interaction with N-sialic acid receptors
- AAV8: Is particularly suitable for liver-targeted therapy through interaction with the LamR receptor
- AAV9: Has broad tissue tropism and can cross the blood-brain barrier and placental barrier
Table 1. Selected AAV Serotypes and Their Primary Tissue Tropisms
| Serotype | Primary Tissue Tropisms | Receptors/Cofactors |
|---|---|---|
| AAV1 | Muscle, neural tissues | Not specified |
| AAV2 | Neural tissues | Heparan sulfate proteoglycan |
| AAV5 | Lung, eye, neural tissues | N-sialic acid |
| AAV6 | Blood cells (T cells) | Not specified |
| AAV8 | Liver | LamR receptor |
| AAV9 | Widespread (CNS, muscle, liver, lung) | Crosses biological barriers |
Applications of Recombinant Adeno-Associated Virus
In biomedical research, recombinant adeno-associated virus (rAAV) vectors are powerful tools for studying gene function and can be used in various methods such as gene overexpression and gene knockdown. Their applications span multiple disciplines:
- Neuroscience: rAAVs are small in size and have strong diffusion capabilities, enabling efficient transduction of neurons and glial cells. Specific serotypes can be used for anterograde tracing (AAV1), retrograde tracing (Retro), and widespread central nervous system transduction.
- Metabolic research: Hepatotropic serotypes (AAV8, DJ) can be used to study liver function and metabolic pathways.
- Musculoskeletal research: AAV1, AAV6, AAV8, and AAV9 can effectively transduce skeletal muscle and cardiac muscle tissue.
- Ophthalmology research: AAV2, AAV5, and AAV8 demonstrate high efficiency in retinal transduction.
Our Collaborative Process
Gene Design and Vector Construction
Our process begins with comprehensive consultation and project planning. Our research team will work closely with clients to select the optimal promoter system, regulatory elements, and reporter gene tags based on the target cell type and expression requirements. We have an extensive library of validated vector backbones suitable for various applications, including gene overexpression, gene knockdown, and regulatory RNA manipulation. At this stage, we carefully consider transgene size limitations to ensure optimal packaging and performance.
Serotype Selection and Optimization
We provide expert guidance to help clients select the most suitable viral capsid for their specific experimental or therapeutic application. For clients unsure of the best serotype choice, we offer screening services using pre-packaged serotype libraries to experimentally determine the most suitable variant for a particular target tissue. Our libraries include natural serotypes and engineered capsids with enhanced properties, such as the ability to penetrate the blood-brain barrier or exhibit cell-type specificity.
Vector Production and Quality Control
We utilize an advanced serum-free suspension culture system for scalable recombinant adeno-associated virus (rAAV) production. This process includes rigorous quality control measures, including:
- Quantification of vector genome titer using ddPCR for absolute quantification
- Measurement of infectious titer to assess functional activity
- Purity analysis to assess the ratio of empty to full capsids
- Endotoxin and sterility testing to ensure product safety
Functional Validation and Delivery
Before delivery, each batch of rAAV preparation undergoes comprehensive functional validation using relevant in vitro models to confirm transgene expression and biological activity. Each shipment is accompanied by a detailed characterization report, including a certificate of analysis, recommended storage conditions, and usage guidelines. Our packaging ensures safe transport and utilizes a cold chain shipping protocol to maintain stability.
Why Choose Our Products?
Creative Biolabs is more than just a supplier; we are your dedicated scientific partner committed to accelerating your biomedical discovery journey. Our competitive advantage stems from the unique synergy of scientific expertise, technological innovation, and an unwavering commitment to regulatory excellence.
01 Scientific Leadership and Expertise
Our operations are led by a team of vectorology experts, many holding PhDs in virology, gene therapy, and molecular biology.
02 Proprietary High-Performance Production System
We have developed and continuously optimize a proprietary, scalable AAV production platform that consistently achieves higher volumetric yields compared to industry standard methods.
03 Quality and Regulatory Compliance
We provide complete documentation, including detailed batch production records and comprehensive quality control release data, ensuring full traceability and compliance with global regulatory standards for preclinical studies.
04 Customization and Flexibility
Our team can tailor the entire production process to the specific needs of your project. We offer custom packaging services for virtually all known AAV serotypes and novel variants, ensuring your vector perfectly matches your experimental design.
Customer Reviews
"The Creative Biolabs team provided exceptional service throughout the entire collaboration project. The recombinant adeno-associated virus (rAAV) vectors they prepared for our neuroscience research exhibited extremely high transduction efficiency in primary neurons, with titers far exceeding our expectations. The packaging was safe and reliable, and the comprehensive characterization data provided with the shipment gave us complete confidence in the quality of the materials. Their technical staff were always happy to answer our questions and provided advice on optimization strategies for in vivo delivery."
— Dr. Elena Rodriguez, Principal Investigator
"As a research team new to viral vector technology, we needed significant guidance in selecting the appropriate serotype and delivery parameters. The scientists at Creative Biolabs provided excellent consulting services and recommended an AAV variant we hadn't previously considered, which significantly improved our transduction efficiency in muscle tissue. The ordering process was simple and convenient, delivery was prompt, and the documentation was extremely thorough. Since then, we have listed them as our preferred supplier for our viral vector needs."
— Dr. Benjamin Carter, Research Assistant Professor
Result Delivery
We are committed to the timely delivery of high-quality recombinant adeno-associated virus (rAAV) preparations and provide complete characterization data:
All rAAV preparations come with comprehensive documentation, including:
- Certificate of Analysis, detailing vector genome titer, infectivity titer, and purity indicators
- Quality control data from electrophoresis analysis, PCR verification, and sterility testing
- Production summary, outlining specific serotype, promoter, and transgene information
- Recommended storage conditions and usage guidelines to maintain vector potency
Frequently Asked Questions
Q: What is the maximum size limit for a target gene (GOI) that can be packaged into an rAAV vector?
A: The packaging capacity of the AAV capsid is strictly limited to approximately 4.7 kilobases (kb) of single-stranded DNA, including the ITR sequences. For therapeutic genes exceeding this limit, we employ strategies such as miniature gene constructs (e.g., mini-dystrophin for DMD) or dual-vector systems. The dual-vector approach involves splitting the therapeutic gene into two separate rAAV vectors (one carrying the 5' end and the other carrying the 3' end), which are then combined in vivo through trans-splicing or homologous recombination to express the full-length protein.
Q: How safe are rAAV vectors for in vivo use, particularly regarding integration and immunogenicity?
A: rAAV vectors are considered one of the safest platforms currently available because they are non-integrating. They primarily exist as episomes, minimizing the risk of oncogenesis due to insertional mutagenesis. Immunogenicity is a major biological challenge; this is typically caused by the host immune system recognizing the viral capsid proteins, leading to the production of neutralizing antibody (NAb) responses and/or cytotoxic T lymphocyte (CTL) responses against transduced cells. We manage this risk by: (1) providing high-purity vectors with low empty capsid rates, (2) recommending serotype screening to select variants with lower pre-existing NAb prevalence, and (3) offering novel engineered capsids designed to reduce immunogenicity.
Q: What is the method for selecting the most effective rAAV serotype for a novel target tissue?
A: Serotype selection is arguably the most critical design element. We recommend performing in vitro and/or in vivo serotype screening to empirically test a small number of the most promising wild-type and engineered capsids (e.g., AAV1, 2, 5, 8, 9, and selected proprietary variants), evaluating them against your specific target cells or animal model. This data-driven approach avoids guesswork and ensures the highest transduction efficiency and minimal off-target effects before proceeding to large-scale production.
Q: How long does transgene expression persist following rAAV transduction?
A: rAAV predominantly persists as episomal DNA in transduced cells, providing long-term expression in non-dividing or slowly dividing tissues that can persist for several months to years in animal models and human patients. The duration varies based on target tissue turnover rate, promoter selection, and immunogenic responses to the transgene product.
Connect with Us Anytime!
rAAV is an outstanding achievement representing the perfect integration of virology, genetic engineering, and clinical medicine. Its remarkable characteristics, including low pathogenicity, non-integrating life cycle, and broad tissue tropism mediated by various serotypes, make it the preferred vector for in vivo gene delivery. At Creative Biolabs, we are not merely spectators in this revolution, but active participants, committed to advancing viral vector technology. If you require further information, please feel free to contact us to request a quote and have an in-depth discussion with our scientists.
Reference
- Bower J J, Song L, Bastola P, et al. Harnessing the natural biology of adeno-associated virus to enhance the efficacy of cancer gene therapy. Viruses, 2021, 13(7): 1205. https://doi.org/10.3390/v13071205 (Distributed under Open Access license CC BY 4.0, without modification.)
