N-glycosylation is one of the major post-translational modifications in nature, and its influence on protein structure and function is very important. N-glycosylation is mainly produced by the co-translational process of the endoplasmic reticulum and is modified by a variety of glycosylases and glycosyltransferases located in the endoplasmic reticulum and Golgi apparatus. Figure 1 shows the biosynthetic process of antibody N-glycosylation. A single N-linked glycan can be connected to N297 in the CH2 domain of each heavy chain. It has a complex dual-antenna structure, in which two arms are composed of three cores. The α1,3 and α1,6 mannose linkages of mannose, such as G0F and G1F, can be extended by the addition of galactose and sialic acid, while the oligomannose 9 structure consists of a 14-saccharide N-glycan precursor in the oligosaccharide Under the action of transferase, it is transferred to the amide nitrogen of the N side chain of the nascent polypeptide, and then is sequentially trimmed by α-glucosidase to form, and the α1,2-linked mannose in Man9 is removed by α-mannosidase I. Glycans can be converted into mannooligosaccharides 5.
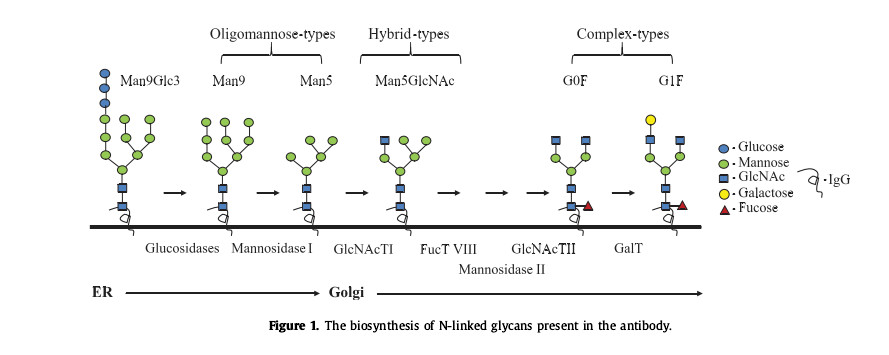
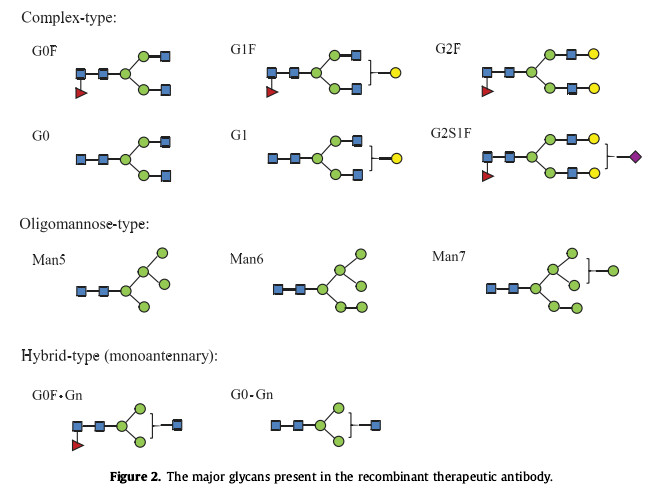
Figure 2 shows the main glycan forms present in recombinant therapeutic antibodies. N-glycans, as the hydrophilic part of glycoproteins, play an important role in protein stability. They protect proteins from proteolysis by maintaining optimal conformation. The effects of aggregation and thermal denaturation. There is also a large amount of data indicating that N-glycans also play an important role in the pharmacodynamics and pharmacokinetics of recombinant therapeutic glycoproteins and antibodies. In addition, N-glycans can also interact with a variety of Glycan-binding protein interactions promote or reduce adverse immune responses to proteins. This article mainly summarizes the impact of N-glycans on protein stability, pharmacokinetics and immunogenicity, providing a reference for optimizing N-glycosylation.
Effect of N-glycosylation on protein stability
N-glycans, as structural components, affect the physical and chemical properties of proteins. Chemical instability involves the formation and destruction of covalent bonds within the protein, such as deamination, oxidation, and peptide bond hydrolysis. Physical instability includes aggregation. and thermal denaturation, etc. These instabilities will lead to accelerated protein degradation or aggregation during drug storage or during patient treatment, reducing drug safety.
(1) Effect of N-glycosylation on the stability of recombinant cytokines and enzymes, such as erythropoietin, interferon-b, interferon-g, ribonuclease, α-galactosidase and tripeptidyl peptide Enzymes, deglycosylation of such substances will lead to increased sensitivity to thermal denaturation and proteolytic hydrolysis. For example, non-glycosylated erythropoietin lacks biological activity in vivo and shows low thermal stability.
(2) The effect of N-glycosylation on antibody stability. In one study, the antibody IgG1-Fc was sequentially deglycosylated with several exoglycosidases, including sialidase, β-galactosidase, β-hexosaminidase and α-mannosidase to generate proteins with different glycoforms. The results showed that the terminal galactose residue has minimal impact on the thermal stability of IgG1-Fc and the thermal stability of the CH2 domain after deglycosylation. The transition temperature is about 5℃-8℃ lower than that of glycosylated antibodies, and has lower thermal stability, proteolysis and aggregation rate.
(3) Glycosylation plays an important role in proteolytic stability. Deglycosylated antibodies show high susceptibility to 3 proteases (human neutrophil elastase, papain, and pepsin). Full galactose Kylation and sialylation increase the sensitivity of antibodies to papain digestion.
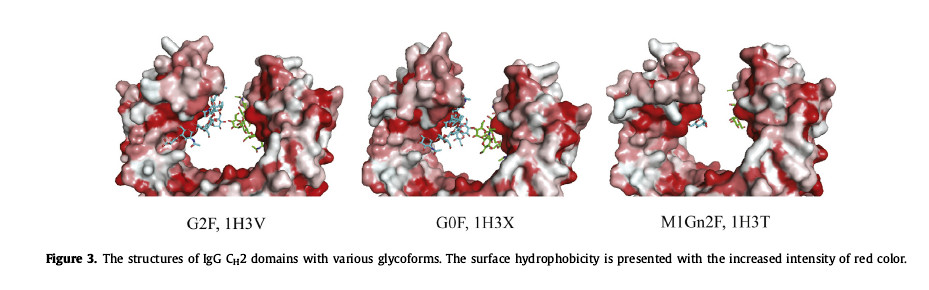
(4) Large conformational changes can occur in the polypeptide ring. The C’E ring contains N-glycosylation sites. After glycosylation, the CH2 domain will be mutated, resulting in a “closed” conformation. Since the glycan is located in the protein near the hydrophobic portion of the backbone, so removal of the N297 glycan results in strong hydrophobic interactions with the CH2 domain (Figure 3).
Effect of N-glycosylation on protein pharmacokinetics
Pharmacokinetics are directly related to the clinical effects of therapeutic proteins, with rapid clearance from serum leading to significantly increased degradation in the liver and limiting the amount of protein drugs reaching target cells. N-glycans play an important role in regulating serum concentrations of recombinant proteins, a role that is often variable and depends on the expression system or cell culture conditions.
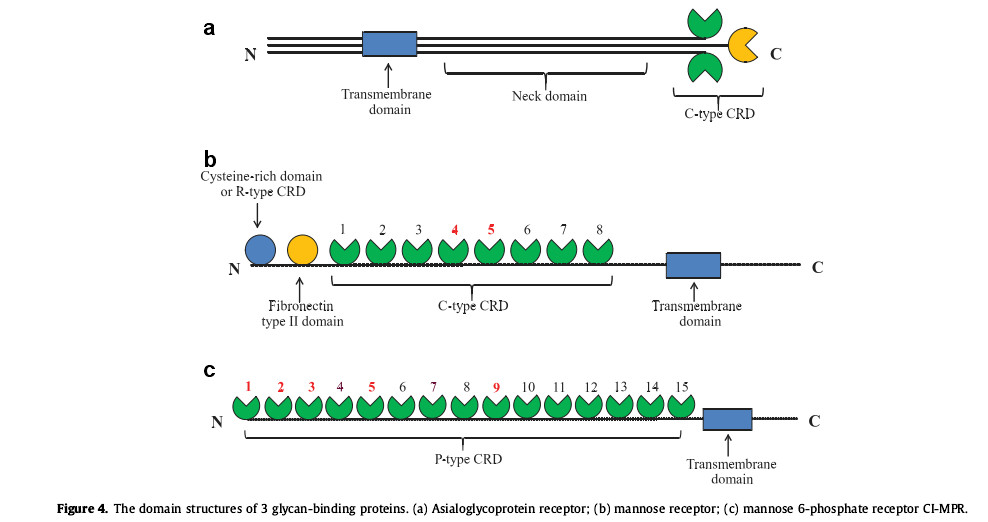
(1) Sialyl glycans have a significant impact on the isoelectric point properties of glycoproteins. Therapeutic proteins containing a higher percentage of sialylated proteins contain a large number of carboxyl groups, so they have a lower isoelectric point. Since they cannot be combined with asialyl Glycoprotein receptor (ASGPR) binding and therefore exhibits a long serum half-life. Human ASGPR is composed of two type II transmembrane glycoprotein subunits, called asialoglycoprotein receptor-1 (Asgr-1) and Asgr-2. The two subunits act as hetero-oligomers or homo-oligomers. Existing in polymeric form, such as heterotrimeric form (Fig. 4a), each subunit contains a C-type carbohydrate recognition domain (CRD) and a stem region, which serves as the C-terminal extracellular domain and also contains a transmembrane region and the N-terminal cytoplasmic domain of endocytosis signaling. This protein receptor is mainly expressed on the surface of hepatocytes, binds to terminal galactose or N-acetylgalactosamine, and has a higher affinity for sugars with highly branched structures, such as three-antennary or four-antennary glycans. Physiological findings indicate that Asgr-1 is important for maintaining normal circulating levels of coagulation factor VIII (FVIII) in plasma, suggesting a role in regulating blood coagulation and thrombosis.
(2) ManR also has the same role. ManR (CD-206, Mrc1) is a 175kDa type I transmembrane glycoprotein, an R type CRD containing N-terminal cysteine, and then a fibronectin type II domain and 8 C type CRD as an extracellular domain, followed by a transmembrane domain and a C-terminal cytoplasmic tail (Fig. 4b). ManR can interact with proteins containing oligomannose or hybrid glycans, and then absorb therapeutic proteins from serum into intracellular lysosomes through receptor-mediated endocytosis, where they are excreted in various proteases and glycosides. Degraded by enzymes. In mouse experiments with antibodies containing oligomannose or fucosylated mannose, the researchers confirmed that human serum containing oligomannose antibodies had a shorter half-life.
(3) The impact of N297 glycan on antibody PK. Although deglycosylated IgG has lower stability as mentioned above, removal of glycosylation through enzymatic deglycosylation or mutagenic sugar chains has a negative impact on the antibody. There is little effect on pharmacokinetics. However, a recent study showed that a novel 2-nonglycosylated N297G mutant had a relatively high clearance rate, so this result remains to be studied.
Effect of N-glycosylation on protein immunogenicity
In addition to pharmacokinetics and stability, immunogenicity is another important property related to efficiency and safety. Immunogenicity can lead to adverse clinical reactions to therapeutic proteins, such as allergic reactions and reduced efficacy. There are many factors that affect immunogenicity, including protein sequence variation, glycosylation, denervation, and aggregation. (1) Recombinant proteins with non-human glycans are immunogenic and may be pre-recognized by the immune system. Such glycans include α1,3-linked galactose (α-Gal), N-glycolyl neuron amino acid (Neu5Gc), β1,2-linked xylose, and α1,3-linked fucose, which are commonly found in recombinant glycoproteins expressed from non-human cells or other non-mammalian species. Adverse clinical immune responses to cetuximab have been reported to be related to the antibody N-glycan terminal α-Gal produced in mouse myeloma cell lines.
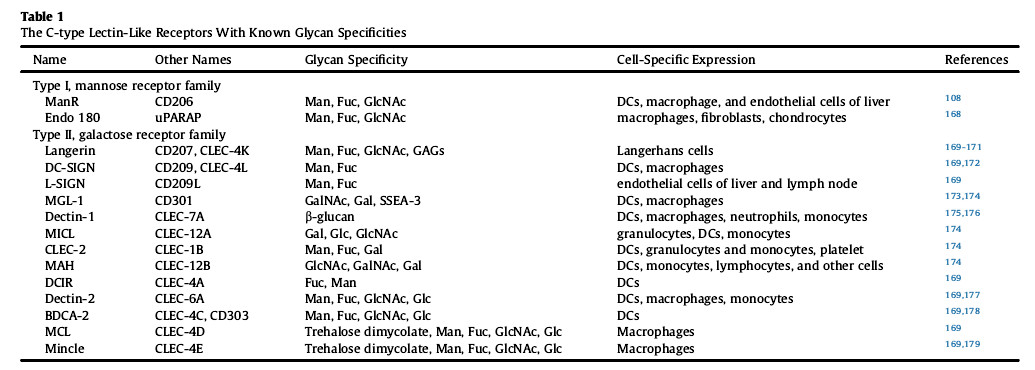
(2) In addition to non-human glycans, human N-glycans in recombinant proteins may contribute to immune regulation. Many glycan-binding proteins exist on human immune cells, especially cells of the innate immune system, including diatom proteins, galectins, and C-type lectin-like receptors (CLR or CTLR). CLRs containing C-type lectin-like domains are located on antigen-presenting cells of dendritic cells and can specifically bind to glycans such as oligomannose and fucosylated complex glycans (Table 1) to enhance T cells. tolerance.
(3) Studies have shown that enhanced tolerance needs to be mediated through the mannose 6-phosphate receptor (MPR), but only 1-3, 5 and 1-3 of the cation-independent mannose 6-phosphate receptor (CI-MPR) Only position 9 can bind to the mannose containing glycoprotein to bind to the 6-phosphate receptor surface (Figure 4c).
Summary: The presence of N-glycans increases the hydrophilicity of the protein and protects amino acid residues that are prone to aggregation, thereby enhancing the stability of the protein. Based on the fact that N-glycans can also affect serum half-life and immune response, during the research and development process, various therapeutic proteins should be extensively studied and optimized to improve their safety.
Support of Creative Biolabs
Creative Biolabs is a leading customer service provider in the field of therapeutic glycoprotein development. Here is a simple list of our custom services.
- MALDI-TOF MS
- HPAEC-PAD
- Cell Line Glycoengineering Services
- Strategies of Genetic Glycoengineering
- Glycan Profiling
Reference: QunZhou., et al. “The Mechanistic Impact of N-Glycosylation on Stability,Pharmacokinetics, andImmunogenicity of Therapeutic Proteins.”Journal of PharmaceuticalSciences.. 2023;8(3):199-221.
