Therapeutic Glycoprotein Development Services
Therapeutic glycoproteins have become the fastest growing fields in the pharmaceutical industry. With first-in-class technologies and experienced scientists, Creative Biolabs has successfully developed versatile glycoprotein production platforms. Based on these cutting-edge platforms, we can provide our worldwide clients with the largest and most diverse portfolio of standard or custom therapeutic glycoprotein services. As your best partner, Creative Biolabs is very glad to revolutionize your particular project and promote your success based on the values of quality, timing, and price.
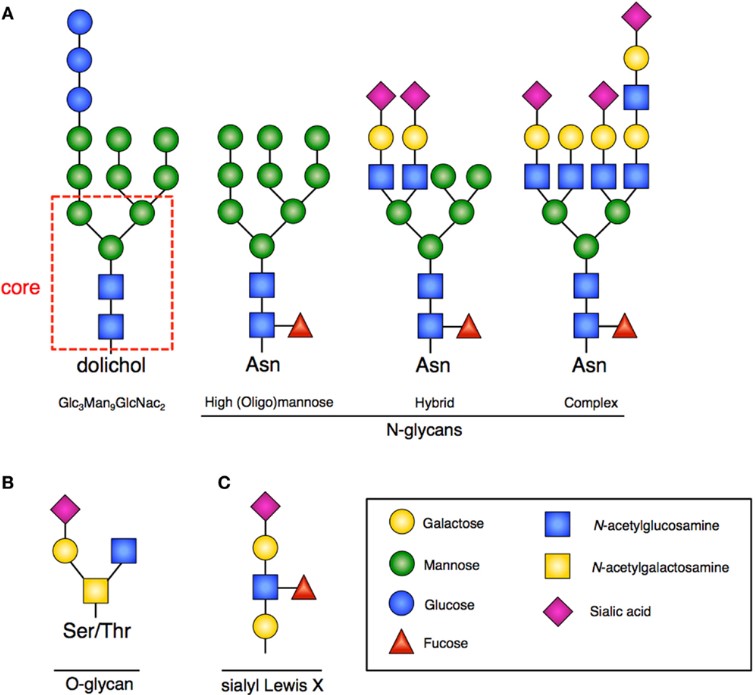 Fig.1 Structure of glycan.1, 3
Fig.1 Structure of glycan.1, 3
Background of Therapeutic Glycoprotein
Therapeutic glycoprotein is a type of proteins that contain oligosaccharide chains (glycans) covalently attached to amino acid side-chains. Glycoproteins are generated by glycosylation, which is a very critical modification of therapeutic proteins, known to significantly modulate yield, bioactivity, solubility, stability against proteolysis, immunogenicity, and clearance rate from circulation. It is documented that all of the key molecules involved in the immune response are glycoproteins and many therapeutic proteins like vaccines, antibodies and enzymes require glycans to have full biological activity.
Therapeutic Glycoprotein Development in Creative Biolabs
Creative Biolabs has developed versatile Glyco-engineered Systems for Therapeutic Glycoprotein Development, which are more suitable for the production of defucosylated antibodies and fully humanized glycoproteins. We provide a wide range of cells from various origins, including yeast cells, plant cells, and various mammalian cell lines. Our advanced technology overcomes shortcomings in non-mammalian expression systems. Our repertoire of mammalian cells includes a variety of cell lines such as Chinese hamster ovary (CHO) cells, baby hamster kidney (BHK) cells, human fibrosarcoma cells, human embryonic kidney (HEK293) cells, and human HT-1080 cells, ensuring a suitable match for your specific project requirements.
In addition, we improve the cell culture media for our glycoprotein production cells. Serum is essential for cell culture in vitro, but it is expensive and exists a high risk of adventitious agents. To substitute for serum in cell cultures, some adventitious agents such as albumin, transferrin, and insulin have been used for glycosylated proteins production. Our serum-free media (SFM) with no animal-derived components has become an industry standard and is preferred for large-scale production with low cost. The proprietary cell culture media can support the growth of most industrially relevant cell lines with high cell densities (>10 million cells/mL) and specific growth rates.
Proprietary Cell Lines in Creative Biolabs
The specific human cell lines are generated by glycoengineering, which are more productive and cost-effective.
-
HighSialo-Cell line: It is used for the production of biopharmaceuticals with high sialylation and high core fucosylation.
-
Sialo-Cell line: It is used for the production of biopharmaceuticals with gradual adjustment of the sialylation degree.
-
Fuco-Cell line: It is used for the production of biopharmaceuticals with gradual adjustment of the fucosylation degree.
Advantages of our Therapeutic Glycoprotein Development Platforms
Based on our platform, we can produce a variety of glycosylated biopharmaceuticals and optimize the glycosylation to improve activity and/or other properties like bioavailability, stability, and/or immunogenicity for better clinical performance.
-
Consistency (minimized batch-to-batch variations)
-
High efficiency (homogenous, stable and fully human glycosylated biomolecules)
-
Stable protein quality and high yield
-
Cost effective for large-scale production
-
Best after-sale service
Applications of Our Cutting-edge Platforms
-
Hormones (e.g., follicle-stimulating hormone, FSH)
-
Different antibodies subtypes (IgG, IgM, IgA, etc.)
-
Bispecific and defucosylated antibodies
-
Blood factors
-
Enzymes
-
Glycosylated proteins that are difficult-to-express
-
Fusion proteins with extended serum half-life
In addition, our advanced platform based on proprietary glycoengineered human cell lines is also beneficial for the optimization of glycosylation, which may avoid severe immunogenic reactions.
Let's Work Together to Fulfil Your Glycoprotein Project
Creative Biolabs has successfully completed a lot of therapeutic glycoprotein projects. It is exciting to offer our off-the-shelf product portfolio and services to help you get landmark development. We can customize our offering to meet your specific project needs. If you are interested, please contact us for more information.
Published data
The spike glycoprotein binds with high affinity to the host receptor angiotensin-converting enzyme 2 (ACE2), and the coronavirus SARS-CoV-2 uses the surface glycosylated spike protein to enter and infect host cells. Therefore, developing molecules that can interfere with the binding of the SARS-CoV-2 spike protein to ACE2 has the potential to inhibit viral entry into cells. In this study, the authors designed and developed a highly stable spike interactor protein, which was a microprotein embedded in the ACE2 receptor by embedding key spike interactor residues. This design was intended to recognize all coronaviruses that enter the host through the ACE2 receptor. Starting with a set of soluble and stable spike interactors (S-plug), the research team mutated its structure and recombined it through molecular dynamics simulation to produce the microprotein S-plug2, which was highly stable in solution. Subsequently, the researchers further improved the S-plug2 microprotein by adding an arm with a cysteine (Cys) residue at the C-terminus to induce the formation of a Cys lock, resulting in the S-plug3 microprotein, which was more stable than S-plug2. Its binding affinity to both the spike S1 domain and the receptor binding domain (RBD) was enhanced. This study provided an effective basic tool for the development of therapeutics and diagnostics against the SARS-CoV-2 coronavirus.
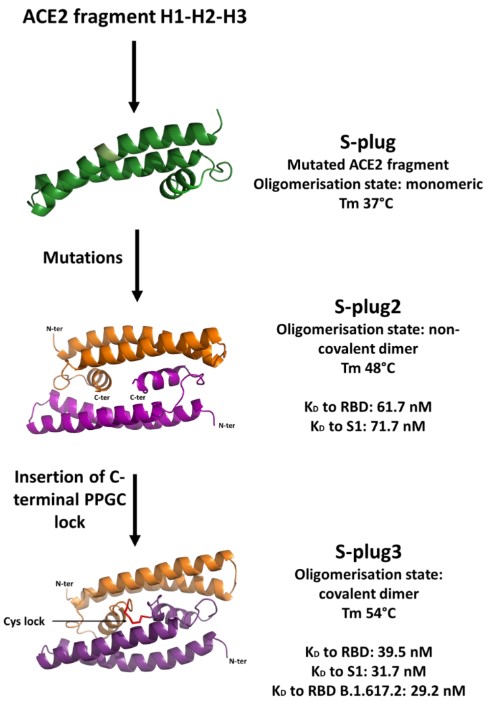 Fig.2 S-plug-based microproteins development process.2, 3
Fig.2 S-plug-based microproteins development process.2, 3
FAQ
Q1: What are the characteristics of your therapeutic glycoprotein production platform?
A1: Our glycoprotein production platform is diversified and highly customizable. We provide efficient glycosylation modification and stable glycoprotein production services. In addition, we have cells from various sources, including yeast cells, plant cells, and a variety of mammalian cell lines, to ensure that we meet the specific needs of your project.
Q2: What are the specific advantages of your SFM in large-scale production?
A2: We provide SFM that is completely free of animal components and is suitable for a variety of industrial-related cell lines. Our culture medium is optimized to support high-density cell growth while reducing production costs and contamination risks, making it very suitable for large-scale production.
Q3: How do you ensure the stability and consistency of glycoprotein batches in large-scale commercial production?
A3: We use advanced glyco-engineered technology to ensure that each batch of glycoproteins has consistent glycosylation modification and high quality. Our cell lines and culture media are strictly quality-controlled and validated to minimize batch-to-batch differences and ensure product consistency and stability.
Customer Review
Successful Development of High Core Fucosylated Antibodies
“As a scientist who has worked in the field of therapeutic glycoprotein research for many years, I am very satisfied with Creative Biolabs' technology platform and service quality. We commissioned them to develop an antibody with high core fucosylation, and the results showed that their Fuco-Cell cell line could very accurately adjust the degree of glycosylation to meet our most stringent requirements for antibody potency and immunogenicity. Throughout the project, Creative Biolabs provided extremely detailed data support, allowing us to have a deep understanding of every link. This successful project laid the foundation for our future long-term cooperation.”
Produce Stable And Consistent Glycoprotein Products
“Creative Biolabs has an advanced glycoprotein production platform and first-class technical service level. Their high-yield cell line and optimized culture medium system greatly improved the production efficiency of glycoproteins, thereby significantly shortening the project cycle. The glycoprotein products finally produced had stable quality and good consistency, which fully met our needs.”
References
-
Lyons, Jonathan J., Joshua D. Milner, and Sergio D. Rosenzweig. "Glycans instructing immunity: the emerging role of altered glycosylation in clinical immunology." Frontiers in Pediatrics 3 (2015): 54.
-
Squeglia, Flavia, et al. "Structure-based development of SARS-CoV-2 spike interactors." International Journal of Molecular Sciences 23.10 (2022): 5601.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 Structure of glycan.1, 3
Fig.1 Structure of glycan.1, 3
 Fig.2 S-plug-based microproteins development process.2, 3
Fig.2 S-plug-based microproteins development process.2, 3

