Unlike the mechanism of action of traditional treatments, immunotherapy is uniquely characterized by its long-term response and survival in efficacy, unconventional delayed response and pseudo-progression in response mode, a new assessment endpoint possibly needed due to its unconventional response mode, and its unique immunotherapy-related adverse effects (irAE) in terms of side effects.
Today, immunotherapy is the hot topic in the oncology field, and particularly immunological checkpoint inhibitors (ICIs) may be the most promising immunotherapy. Different from chemotherapy and targeted therapy in tumor cells, ICIs directly act on the autoimmune system, block the binding of immune checkpoints (such as PD-1) and their ligands, and restore T cell activation and proliferation to kill tumors.
1. Long-term response and survival
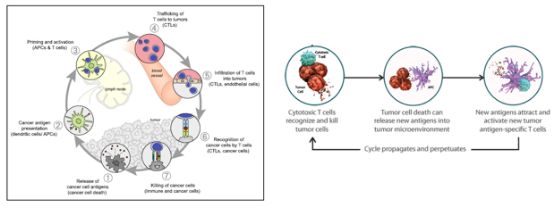
The anti-tumor immune response is a process of repeated cycles that can continue to increase and expand over time, and T cells further memory tumor antigens and convert to mature memory T cells. Even if tumor antigen stimulation does not exist, it can also kill tumor cells. The long-term recognition and immune memory help maintain the anti-tumor immune response, resulting in long-term survival benefits.
Figure 1. Anti-tumor immune response continues to circulate (Daniel S Chen, 2013)
There have been many clinical studies that further exemplify the long-term response and survival of ICIs. The survival curves of clinical trials of immunotherapy have a “long tail” shape, and patients who have not progressed have a longer duration.
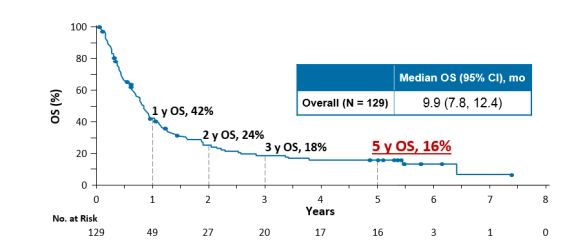
Long-term survival: The longest survival data for lung cancer immunotherapy follow-up comes from the CA209-003 study of nivolumab. The 5-year follow-up results show that the 5-year survival rate of patients treated with nivolumab monotherapy for advanced NSCLC is as high as 16%, improved by 3 times compared to the traditional treatment.
Figure 2. OS benefits in the CA209-003 study (Scott Gettinger, 2018)
In addition, a 3-year follow-up of the Nivolumab global phase III clinical trial, CheckMate017/057, showed that the 3-year survival rate of second-line nivolumab in squamous or non-squamous advanced NSCLC patients was 16% and 18%, respectively.
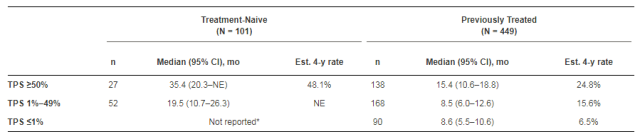
The 4-year follow-up of the Keynote-001 study, published this year at ASCO, showed that the PD-1 inhibitor pembrolizumab improved the 4-year survival rate of patients with advanced NSCLC compared with docetaxel. In addition, 101 patients in the study received first-line treatment of which the estimated 4-year survival rates of first-line and posterior-line treatment were 27.2% and 16.4%, respectively. Patients with PD-L1 positive NSCLC, whether in primary or posterior line, can benefit from OS in pembrolizumab therapy.
Figure 3. OS benefits for different PD-L1 expression levels in the Keynote-001 study (Enriqueta Felip, 2018)
Long-lasting response: 75% of patients in the CA209-003 study did not receive any treatment after nivolumab and did not progress at the last follow-up. In the CheckMate 017 study, the 3-year duration of response (DoR) for patients with squamous NSCLC using nivolumab was 25.2 months, and 26% of patients were still in sustained remission at 3 years of follow-up.
In the CheckMate 057 study, DoR prolongation of non-squamous NSCLC patients with nivolumab increased with a follow-up period (DoR was 17.2 months at 1 year and increased to 18.3 months at 3 years), and 23% patients remained a sustained response at 3 years of follow-up.
The CheckMate 003/Keynote-010 study showed that PD-1 inhibitors were discontinued after 2 years of treatment, and most patients continued to respond. The CheckMate 069 study using nivolumab in combination with ipilimumab in the treatment of melanoma showed that the treatment response can persist for a period of time even if the drug is discontinued due to toxicity.
2. Unconventional response mode
Traditional treatments for cancer can result in three tumor volume or radial changes: tumor volume or single diameter is shrinking (effective); tumor growth (disease progression); tumor volume or radial line is unchanged (stable). Compared with the original three possibilities, two modes of response including delayed response and pseudo-progression may be added during immunotherapy.
Delayed response: Clinically, the treatment response that occurred after 12 weeks of treatment was classified as a delayed response. The effect of chemotherapy drugs is rapid, and the clinical efficacy after clinical administration of 1-2 rounds of chemotherapy drugs can directly predict the therapeutic effect after completing all chemotherapy regimens. Due to the unique mechanism of action of immunotherapy, it may not immediately induce measurable tumor shrinkage after treatment but after several weeks to several months to take effect. In general, the proportion of delayed response to immunotherapy is low. For example, CheckMate 017/057 and Keynote-001 studies show that, on the whole, the time of onset of immunotherapy is basically the same as that of chemotherapy with a mean value of about 2.2 months.
Pseudo-progression: After immunotherapy, some patients may observe a preliminary increase in the size of the tumor or a new lesion after radiological examination. It is confirmed by biopsy that necrosis or inflammatory cell infiltration is accompanied by a decrease in tumor burden, and then the lesion continues to shrink. This unconventional clinical response is considered a pseudo-progression.
Similarly, pseudo-progression is not common in immunotherapy. A 3-year retrospective study of patients with advanced NSCLC who received PD-1 inhibitors found that 50 (9%) of 228 patients had pseudo-progression. In the AK study, 3.6% of patients developed pseudo-progression, and 7% of target patients who continued to receive atezolizumab after progression continued to recede (with a 30% retreat after progression), and 49% of patients had stable target lesions after continued use of atezolizumab. A comprehensive analysis of CheckMate066\067 showed that 28% of patients with melanoma who continued to receive nivolumab after progression had a target lesion retraction >30%.
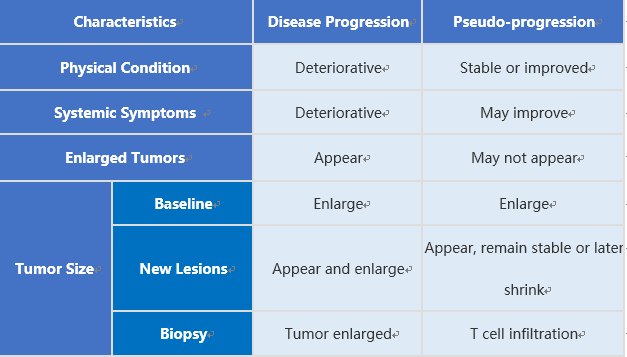
We can identify the progression of disease (PD) and pseudo-progression in terms of physical status, systemic symptoms, tumor-enhancing symptoms, tumor size (baseline, new lesions, biopsy). For example, tumor lesions find enlarged through iconography, but the patient’s PS score has not decreased, and the symptoms have not worsened, then it may be a pseudo-progress. In addition, by biopsy it can be found that the enlarged lesion of PD is a proliferating tumor cell, and the pseudo-progression is the infiltration of T cells.
Table 1. Differences between disease progression and pseudo-progression
3. Appropriate evaluation criteria
Due to the characteristics of tumor immunotherapy, the efficacy of tumor immunotherapy is evaluated according to the traditional efficacy evaluation system (WHO standard and RECIST standard), and the conclusion that treatment is ineffective is often obtained because there is no obvious tumor change. For example, the PD is determined using the RECIST standard, and the tumor is further reduced after continued treatment. Therefore, the continued use of traditional WHO standards and RECIST standards has been unable to adapt to the new tumor treatment methods of tumor immunotherapy. Tumor immunotherapy requires a more reasonable and more feasible new evaluation criteria.
Currently, the irRC standard and the iRECIST standard are commonly used to evaluate tumor immunotherapy, which are improved from the WHO and RECIST standards, respectively. When the target lesion progresses and new lesions appear, they all need to be reconfirmed before evaluated as PD. The irRC standard requires reconfirmation of the iconography PD for at least 4 weeks; the iRECIST standard requires reconfirmation of the iconography PD within 4-8 weeks.
However, these two evaluating standards still have limitations. For instance, the irRC standard has measurement variability, which quantifies tumor burden by two-dimensional measurement, and the 25% increase is defined as PD, but the two-dimensional measurement has variability. Another limitation is caused by the confirmation of the minimum time range of PD by the irRC or iRECIST standards. Since the time range for pseudo-progression and subsequent responses may be broader than currently assumed, pseudo-progression may occur after diagnosis of irRC-PD or iRECIST-PD.
Reference
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013 Jul 25;39(1):1-10. doi: 10.1016/j.immuni.2013.07.012. PMID: 23890059.
Gettinger S, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol. 2018 Jun 10;36(17):1675-1684. doi: 10.1200/JCO.2017.77.0412. Epub 2018 Mar 23. PMID: 29570421.
Enriqueta Felip, et al. 4-year overall survival for patients with advanced NSCLC treated with pembrolizumab: Results from KEYNOTE-001. Journal of Clinical Oncology 36, no. 15_suppl (May 20, 2018) 9030-9030. DOI: 10.1200/JCO.2018.36.15_suppl.9030