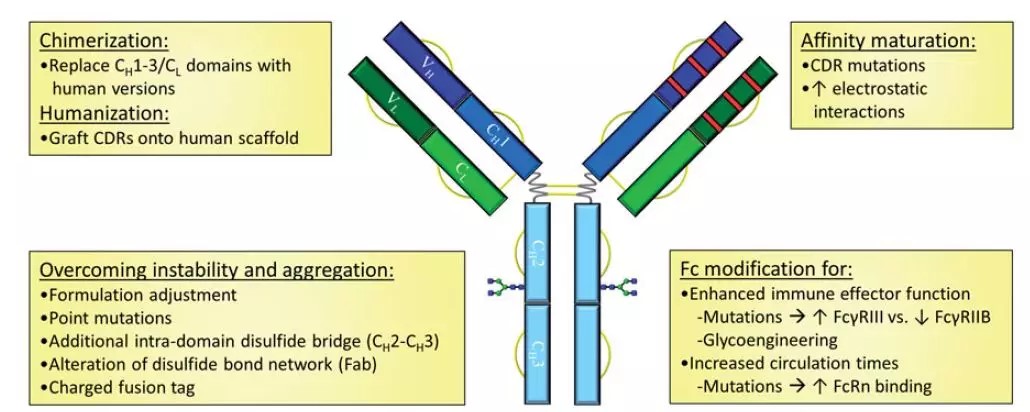
In the process of discovering monoclonal antibodies, after screening antibody libraries to obtain excellent candidate molecules that can specifically bind to targets, it is necessary to enhance certain pharmacological and other functions through certain engineering techniques. Various strategies can improve the kinetic functions of antibody drugs, including affinity maturation, chimerization or humanization, and Fc modification. In order to improve the drug-formability and stability of antibodies, there is a need to improve the stability and aggregation of antibodies, as well as to reduce immunogenicity, some of which can also be achieved by computer tools.
1. Chimerization or humanization reduces immunogenicity
In the early stage, when the mouse antibody enters the human body, it will respond to the human immune system and produce a human anti-mouse antibody (HAMA) response, inducing the hypersensitivity reaction or the effect of the mouse antibody to be neutralized by the anti-mouse antibody. The solution to this problem is to bring the antibodies closer to human antibodies by chimerization or humanization. A human-mouse chimeric antibody is a mouse antibody variable region gene fragment that is ligated to a human antibody constant region gene. Humanized antibodies are transplanted into the CDR region of the mouse antibody to the corresponding site of human antibodies so that humanization can reach more than 90%.
With the development of computer-assisted simulation technology, humanized antibody technology has entered the second generation. Its strategies include part of CDR transplantation, in which part of the CDRs necessary for antibody binding to antigens are grafted onto human antibody frameworks to obtain less immunogenic humanized antibodies; Surface remodeling, referring to the mosaic/remodeling of surface residues of mouse CDRs and FRs, similar to the profile of human antibody CDRs and the form of human FRs; transplantation of specific decision regions, in which the SDR formed by the key amino acid in the antibody that closely interacts with the antigen is transplanted to the corresponding position of the human antibody. In addition, in order to ensure the affinity of antibodies, the researchers also proposed strategies for CDR compensation, positioning retention and template replacement.
Although the humanization of non-human antibodies is still common today, the emergence of transgenic mice and, more importantly, the full human (synthetic) antibody library has reduced the need for these strategies. However, any therapeutic monoclonal antibody is immunogenic regardless of its source. Therefore, this question should always be considered when assessing the patient’s risk-return rate.
2. In vitro affinity maturation
For therapeutic antibodies, an increase in antibody affinity can help reduce dosage and toxic side effects. The in vitro affinity maturation strategy of antibodies is derived from the in vivo antibody maturation process (Figure 1). In this strategy, a small-capacity library of concentrated mutations and specific site mutation is constructed or the mutations are introduced into the variable regions of the original antibody, usually CDRs, and high-affinity antibodies are screened by surface display technology. Particular emphasis needs to be placed on the unique role of CDR H3 in antigen recognition and potential conformational changes. At the same time, the surrounding FR area should also be taken into account. In general, 10-fold improvement in affinity can be obtained.
In order to mimic the adaptive immune system, an SHM in vitro mammalian system, mediated by recombinant AID, is developed with human variable sequence rearrangement, cell surface display and weak binding separation. Low-affinity monoclonal antibodies are preliminarily identified to produce a low pM affinity in SHM in vitro. In SHM, Ig gene insertion and deletion enhance in vivo and in vitro affinity maturation.
Figure 1 Engineered technology enhances antibody pharmacology and stability
Computer tools also apply to the maturation of affinity. Early computer simulations focused on ionic interactions and single mutants to increase 10 to 100 times higher affinity of mAbs (such as cetuximab and bevacizumab). Another combination method based on CDR3 randomization and rational design increased the affinity of low μM scFv by 450 times.
Not all therapeutic applications are eager for a high affinity for optimal efficacy. In the context of cancer, high-affinity binders are bound by “binding site barriers” and can only be located at the edge of the tumor. In another example, anti-transferrin receptor mAbs can only deliver their payload across the blood-brain barrier (BBB) in the blood with their affinity moderate. In both cases, mAbs with lower affinities are still able to bind to the target but are subsequently released, enhancing the ability to penetrate the tumor and BBB.
3. Fc modification enhances effector function and half-life
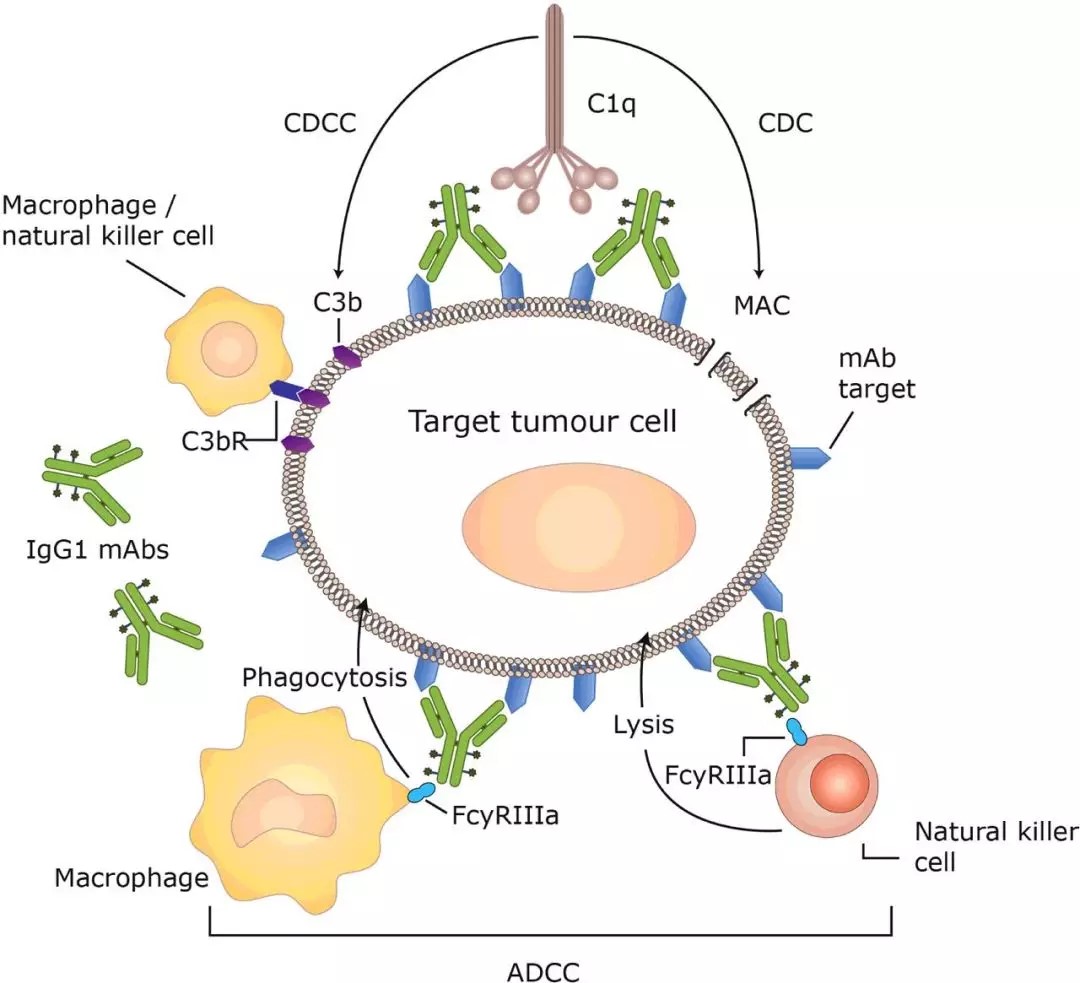
Modification of the Fc region can have a profound effect on the pharmacological effects of a particular antibody. Importantly, the activity of therapeutic mAbs directed against tumors is dominated by activation and inhibition of the Fcγ receptor (FcγR), FcγRIII, or FcγRIIB. Studies have shown that cytotoxic mAbs will preferentially bind to FcγRIII, while reducing the involvement of FcγRIIB with greater clinical efficacy. In rituximab, only a small number of amino acids have been replaced, showing enhanced ADCC effects in vitro and in vivo. In addition, changes in glycosylation can also enhance FcR interactions. Using the GlycoMAb technology, the non-core fucose of the mAbs Fc region is expressed equally, and the anti-CD20 antibody GA101 has stronger ADCC effect, anti-tumor efficacy, and B cell depletion than Rituximab. This technology is combined with POTELLIGENT technology and the antibody drug Mogamulizumab has been approved for the treatment of relapsed or refractory adult T cell leukemia or lymphoma.
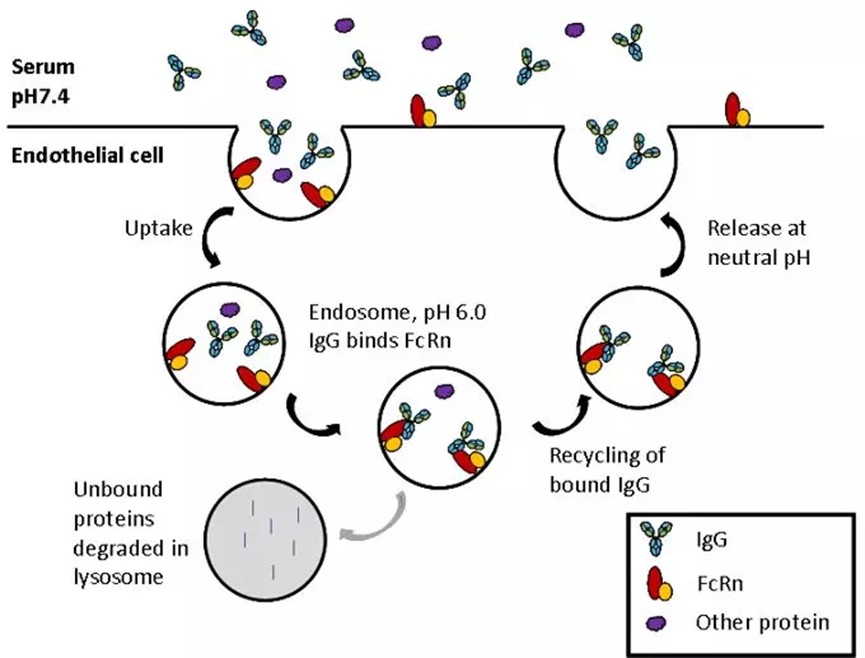
Figure 2 Interaction of Fc fragments of IgG in blood and endosomes with neonatal receptor FcRn affected by pH
Fc also leads to increased blood circulation time. Many studies have investigated the modification of Fc to extend the half-life of the antibody. Due to pH-dependent FcRn interactions, mutagenesis studies focus on replacing Fc amino acids that bind directly to FcRn to increase the affinity at pH=6 rather than pH=7.4. Although Fc modifications to enhanced circulating time may affect Fc’s effector function, studies have shown that mutations in different AAs of bevacizumab and cetuximab lead to more effective antitumor activity in mouse cancer models, thereby validating the strategy of increasing the half-life of therapeutic antibodies. Since then, a large number of favorable mutations have been reported. Unfortunately, the results of animal models are not always related to humans. However, 5-10 times increase in affinity with FcRn usually results in 2-4 times increase in half-life.
Fig. 3 The effect principle of Antibody ADCC and CDC
4. Improve stability and reduce polymerization
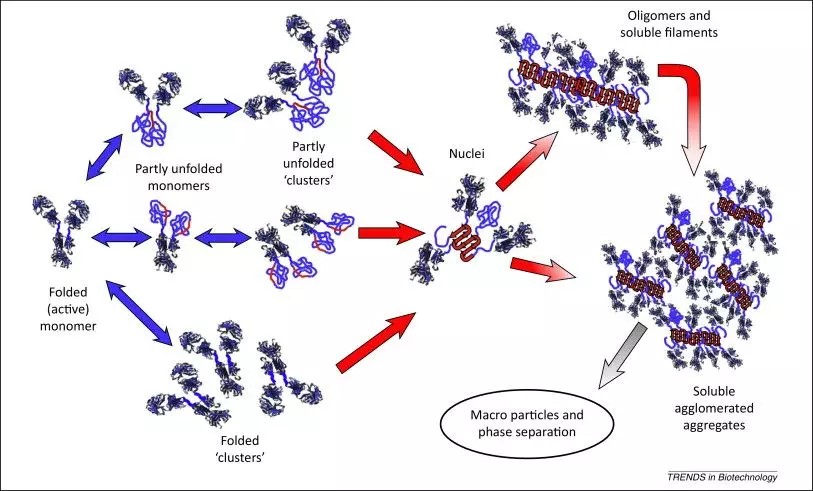
Many therapeutic mAbs have limited thermal and colloidal stability when expressed under non-physiological conditions, making their manufacture and shelf life frustrating. Therefore, some engineering strategies aim to improve the physical characteristics of mAbs to increase their overall efficacy or productivity. The research for reducing polymerization has focused on changing the antibody framework, the antigen-binding loop, and the binding domain-binding interface. More specifically, stabilization strategies include: (a) adjustment of the dosage form; (b) specific point mutations (framework and/or CDRs); (c) addition of additional intradomain disulfide bonds in the CH2-CH3 domain; (d) change the disulfide structure of the Fab region; (e) and/or the charged fusion tag. Similarly, in the development of computer algorithms, rational design and in vitro selection methods can be used to counteract aggregation and predict high viscosity, chemical degradation, and rapid plasma clearance.
Figure 4 Non-natural polymerization of antibodies
The above engineering strategies are mainly focused on improving purified mAbs. In addition, there are other bioconjugation strategies to improve the overall mAb treatment index, each with its own advantages and disadvantages. For example, the coupling of an approved monoclonal antibody to a small molecule drug or a combination of radioisotopes aims to concentrate the vector’s power to the target cell/tissue. Another alternative is a bispecific antibody and CAR-T cell therapy that aims to relocate T cells to cancer for cancer treatment, while nanotechnology uses antibodies as targeting ligands or as diagnostics and/or drug delivery load.
References: Kennedy P J, Oliveira C, Granja P L, et al. Monoclonal antibodies: technologies for early discovery and engineering [J]. Critical reviews in biotechnology, 2018, 38(3): 394-408.