The human immunodeficiency virus (HIV) evades the body’s immune defenses through multiple mutations, and antibodies produced by the host immune system against HIV follow complex and untraceable evolutionary pathways. This complexity makes it difficult for scientists to develop a preventive HIV vaccine that triggers the development of effective antibodies in some HIV-infected individuals.
Now, in a new study, researchers from the Duke Human Vaccine Institute report that they have completed part of a roadmap to effectively neutralize HIV, identifying the steps needed for a crucial class of HIV antibodies to produce and maintain their effective neutralization of the virus. Related research findings were published in Immunity on December 18, 2018, entitled “Inference of the HIV-1 VRC01 Antibody Lineage Unmutated Common Ancestor Reveals Alternative Pathways to Overcome a Key Glycan Barrier.” Corresponding authors are Dr. Mattia Bonsignori and Dr. Barton Haynes of the Duke Human Vaccine Institute.
Picture from Immunity, doi:10.1016/j.immuni.2018.10.015
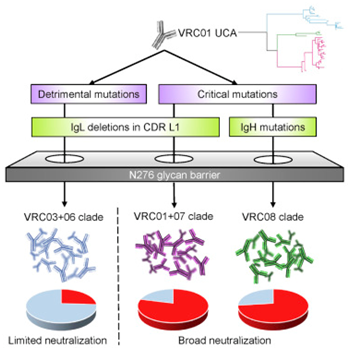
In the new study, Bonsignori, Haynes and their colleagues focused on a specific class of broadly neutralizing antibodies called VRC01 that target the CD4 binding site, a conserved region of the HIV envelope. Such antibody lineages have long been considered a key component of protective vaccine-induced immune responses because they neutralize most of the HIV variants.
”The broadly neutralizing antibodies undergo a long and complex maturation process. They have been extensively studied in this area, but until now we still fail to study their unmutated ancestors, or their origins, because it is very difficult to trace the sequence of many mutations, deletions and changes,”Bonsignori said.
The researchers inferred an unmutated common ancestor of the VRC01 antibody lineage and reconstructed the maturation pathways that lead to the most broadly neutralizing antibodies and those that are harmful to HIV transmission.
Using this roadmap, the researchers found that it’s possible for such antibodies to make strategic detours along their developmental pathways to achieve broad neutralization of HIV. This strategic detour is essentially a shortcut around a major barrier that prevents the previously induced broad neutralization of these antibodies.
”This is where everyone gets stuck. we know that if we can figure out how to use these ancestor antibodies, we’ll be on our way. But we always run into this obstacle: in the early stages of antibody maturation, a particular sugar on the HIV envelope blocks the production of broadly neutralizing antibodies, and everything gets stuck here,”Bonsignori said.
The solution, Bonsignori says, is to bypass the sugar, which means getting around the barrier, rather than trying to break it. He also added that “By reconstructing different pathways to maturity, we found that we can take a simpler path to the end. Now we can use the information to design the immune agents so that they can fully interact with the immune system to get around this barrier.”