In a new study, researchers from the University of Tokyo in Japan revealed the structure of a immune protein called immunoglobulin M (IgM) by using computer image analysis and electron microscopy imaging. It offers the possibility of developing more effective drugs for a series of diseases ranging from cancer to nervous system diseases for the future. The results of the study were published in the October 10, 2018 issue of Science Advances, entitled “The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein.” The authors of the paper are Toru Miyazaki and Satoko Arai of the University of Tokyo.
Bona fide 2D structure of IgM pentamer
What is IgM?
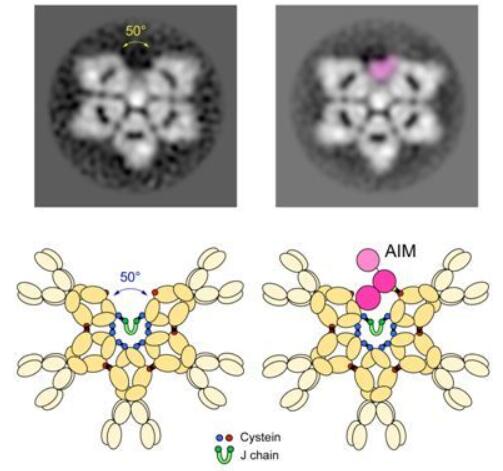
IgM is an important part of the immune system. These researchers validated the structure of native IgM using the human and mouse versions of IgM. They think that IgM should be shaped like an incomplete hexagon, or a pentagon with a wedge-shaped gap. Miyazaki said, “We will have to rewrite the textbook.”
IgM is the first immune system protein produced in human fetuses and is the first molecule protein that responds to pathogen invasion during a lifetime. The structure of IgM was first identified as five-pointed, star-shaped table in 1969 and was updated in 2009 as a five-sided dome or a mushroom cap.
IgM structure is redefined
Miyazaki said, “The original IgM structural model was constructed by observing several IgM molecules through a low-resolution microscope. Now we have a clearer picture and the computer can study thousands of IgM molecules.” When Miyazaki was a medical doctor, he studied a different protein called the apoptotic inhibitor of macrophage (AIM).
Since the correct shape of the IgM was determined, these researchers now understand that the inactive AIM is located in the void of the incomplete hexagon of IgM. The structural association between IgM and AIM means that drugs with the ability to modulate AIM release may be used to develop AIM-based disease treatments. IgM releases AIM when other molecules activate the immune system. AIM proteins of smaller size circulate in the body to remove damaged cells and prevent disease.
In 1999, Miyazaki identified AIM when working at the Institute of Immunology in Basel, Switzerland. Its small size means that AIM is easily excreted from the body and into the urine through the kidneys, so combining it with larger IgM protects the AIM from being removed before it is needed.
AIM is a common molecule in the blood, but it is only active when the body is sick. AIM is known to play an important role in the prevention of obesity, fatty liver disease, hepatocellular carcinoma, multiple sclerosis, fungal induced peritonitis, and acute kidney injury.
This incomplete hexagonal structure is still only a two-dimensional understanding of the IgM structure. Miyazaki and his team continue to conduct further analysis and hope to report the three-dimensional structure of IgM as soon as possible.
References:
- Hiramoto, A. Tsutsumi, R. Suzuki et al. The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein. Science Advances 4: eaau1199 (2018), doi:10.1126/sciadv.aau1199.
- FEINSTEIN et al. Conformation of the Free and Antigen-bound IgM Antibody Molecules, Nature (2006). DOI: 10.1038/2241307a0
- M. Czajkowsky et al. The human IgM pentamer is a mushroom-shaped molecule with a flexural bias, Proceedings of the National Academy of Sciences (2009). DOI: 10.1073/pnas.0903805106