Chimeric antigen receptor (CAR) T cells have hit the cancer immunotherapy area in the past few years.
‘A CAR therapy for cancer, using a technique called adoptive cell transfer, has been approved by the US Food and Drug Administration for use against acute lymphoblastic leukemia. T cells are removed from a patient and modified so that they express receptors specific to the patient’s particular cancer. The T cells, which can then recognize and kill the cancer cells, are reintroduced into the patient. Modification of T-cells sourced from donors other than the patient is also under investigation’
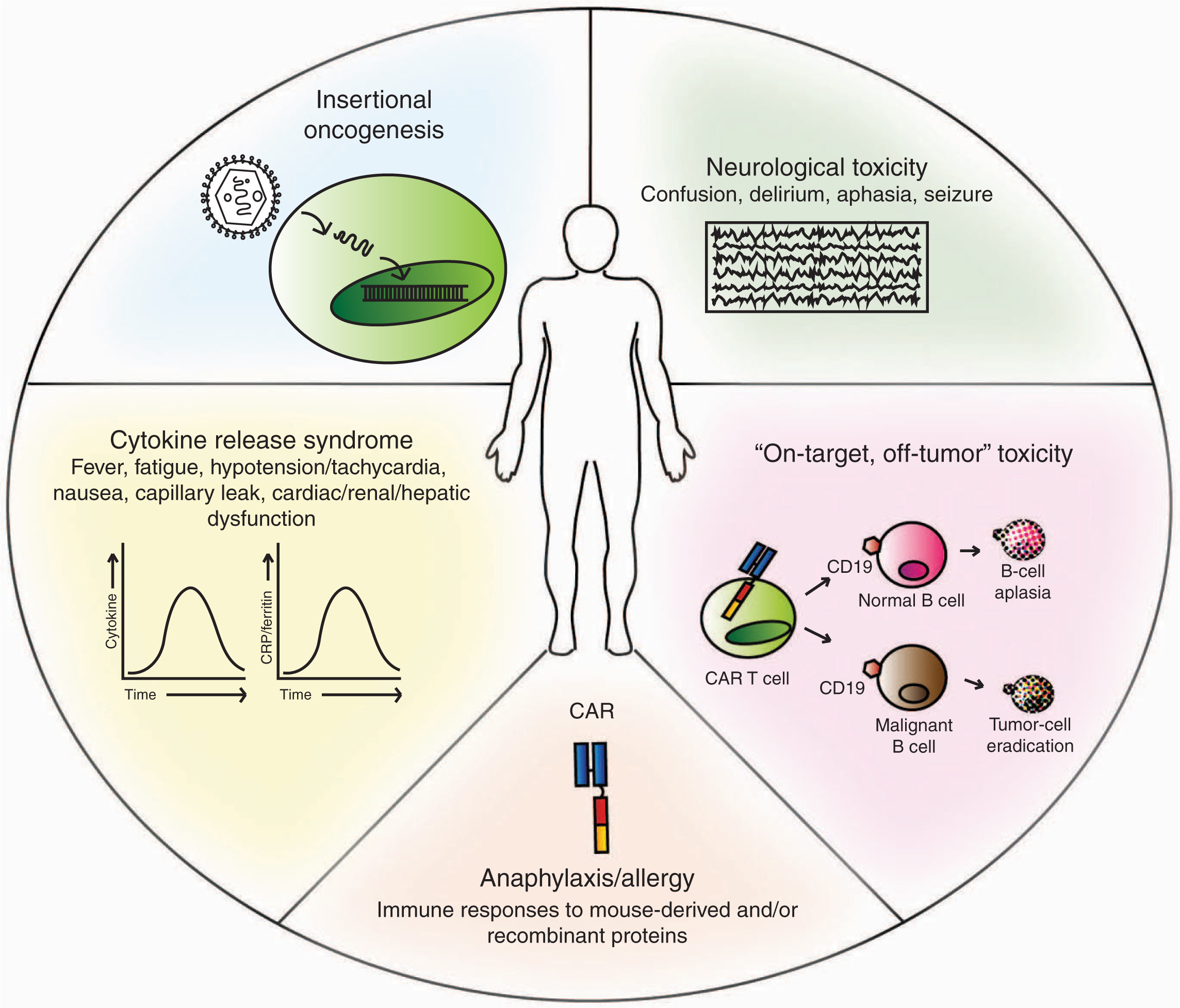
Be that as it may, CAR T-cell therapy can’t exist alone since T cells are generally combined with patient preconditioning as chemotherapy or systemic cytokine, which is related to toxicity. Thus, complete CAR T-cell treatment not only includes the well-known elements but also includes the CAR T cell with largely unknown toxicity. In terms of the latest report of side effects of CAR T-cell therapy, current preclinical animal tests of CAR T-cells may not be suitable for the analysis of CAR T-cell toxicity profiling. Therefore, CAR T cell validation is explored in the use of preclinical models to examine its potential to predict the possible toxicities driven by CAR T-cell.
Generally, most of preclinical trials on CAR T-cell function mainly discuss specificity verification and potency of antitumor activity. The specificity of CAR T-cell has been proved through in vitro assays that T cells with a specific CAR exhibit a different functional response between target antigens and nonspecific antigens. The capability to redirect T-cell effector function without HLA-restriction. Accordingly, allogeneic T-cells are easy to examine this function without matched targeting cells. As the result of allo-recognition, background activity generally plays the same role in cells transduced with unrelated CARs and nontransduced T cells, which is usually able to measure in vitro. Such assays indicate CAR function and help translate into the CAR T -cells animal test system. What’s worth to mention is that background allo-recognition has not been related to antitumor activity in vivo according to a lot of xenograft tumor studies. As for the clinical translation, information on the specific agent is required for the use in the clinical trial, meaning that the only way of assessing the actual clinical agent turns into being the tests in immune-compromised mouse models. However, due to the disability of the residual elements in the mouse immune system, it is limited in spite of the success of human T-cell engraftment in most immune-compromised mice.
Be that as it may, there still exist some studies showing efficacy of CAR T-cell function using animal tests. However, transduced T cells can drive a xeno-graft versus host disease in 50 days. The appearance of xeno-graft versus host diseases is related to persistence of transduced cells, which is affected by the cytokine conditions. Eventually, this limits the ability to evaluate CAR T cells’ long-term effects in this model.
On the other hand, most of these studies involving CAR T -cells targeting human antigens are restricted to expressing the human tumor cells used in mice models. Thus, healthy tissue cannot be investigated when antigen’s targets may be expressed on normal.
To validate this, immune-competent mouse models have been used with the target antigen of mouse homologue. However, the final interactions of ‘mouse CAR-mouse homologue’ may differ from the equivalent human scenario since these models provide additional information on CAR T-cell function with background antigen expression. Another method is to take advantage of transgenic animals to express the human antigen controlled by a physiologically relevant promoter where it is possible to test the CAR specific for the human antigen.
As a matter of fact, the most efficient and common assay belongs to the BaseScope™ Assay. It is a current expansion to the RNAscope® Technology item portfolio, propelled in mid-2016. With the same creative innovation and exclusive test configuration, the BaseScope™ Assay is also refined to recognize as few as 1-4 Z sets.
This capable in situ hybridization innovation enables the particular discovery of changes, short target arrangements, exon intersections, and profoundly homologous groupings, with single atom recognition affectability in a wide range of tissues, tests, and species. In a word, with the promising future of CAR -T cells therapy, these combined methods will be used frequently to demonstrate the function of CAR T in hematologic tumor and CAR T in Solid Tumors, or even more.