Most proteins have a tendency to form polymers, which are accompanied by slow polymer formation when they are produced, stored, transported, and injected into a patient. There are two reasons for why to control the content of monoclonal antibody polymers. On the one hand, it can reduce impurities and increase purity. On the other hand, the presence of polymers in the treatment process will not only reduce the effect of the drug, but also lead to the immune response. In the experiments on animal immunogenicity, it was found that the proportion of natural structures in the polymer and the size of the polymer determine the immunogenicity of the polymer. Therefore, some scholars concluded that the immunogenicity of the polymer is determined by repetitiveness of the surface antigenic epitopes.
The production of polymer is complex. In simple terms, monomeric protein polymer is a stable complex composed of two or more proteins. The formation process includes the destruction of the natural structure of the protein and the formation of a reversible self-polymerization, the reversible self-polymerization becomes irreversible due to the conformational change, the addition of other monomers makes the polymer larger and the polymers interact with each other to form soluble polymers with the size increases once again, and the soluble polymers are separated from each other, resulting in the formation of insoluble polymers.
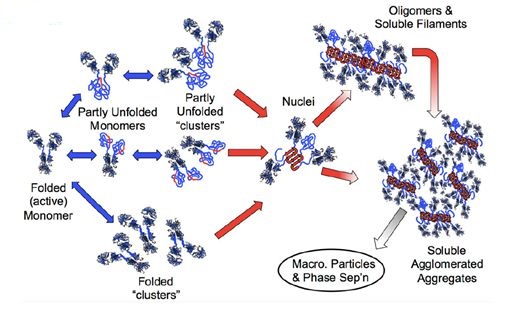
Polymers are usually linked by strong non-covalent binding forces and require a certain degree of conformational changes (misfolded or unfolded) to exhibit key amino acid sequences (also known as “hot spots”, which have strong ability to form irreversible polymers, and are the core of the polymers), thereby aggregating different monomers, which for sure does not mean that no polymer is formed when properly folded. Monoclonal antibodies can form different forms of polymers, as shown in Figure 1. The red area indicates “hot spots”, the blue double-headed arrow indicates a reversible process, the red one-way arrow indicates an irreversible process. Misfolding, unfolding, and correct folding of the Fab and Fc segments of the monoclonal antibody can all form polymers, and they do not dissolve when the soluble polymer splits. However, there is neither definite method nor specific measure to determine in which way does a protein form a polymer, but the content of the polymer is not impossible to be reduced.
Pathway of Monoclonal Antibody Forming polymers (Source: reference )
An amino acid sequence or residue with the tendency to form a polymer, i.e. a “hot spot”, exists not only at a certain position of the protein. Under normal circumstances, the amino acid sequence or residue is highly hydrophobic, low-charged, and easy to form β-sheets. That is, the force that normally promotes the folding of the protein also results in the formation of a polymer. If the “hot spots” exposed in the amino acid sequence can be accurately located and modified to cause repulsion between the proteins, the inherent tendency of the polymers will be affected. However, this genetic approach does not necessarily work because there is no accurate prediction and determination of whether “hot spots” are actually exposed on the surface of proteins and whether they are involved in the formation of polymers.
For monoclonal antibodies, modification of the complement regions (CDRs) of their variable regions, such as facilitating the amino acid mutation at the CDRs edge, and adding glycosides near the CDRs can all increase the resistance to formation of the polymer. In addition, the change in the protein charge is also a measure of the formation of resistance polymers, such as amino acid residues exposed by mutation and the addition of a charged polypeptide at the end of the antibody. Studies have pointed out that the existence of strong negatively charged amino acids (such as peptides) can enhance the electrostatic repulsive force between polymers, so that the formation of a polymer can be suppressed. Oxidation of methionine residues has an important influence on the propensity of many proteins to form polymers. To prevent the oxidation of methionine residues, freezing is usually the choice of antibody storage and transport, which can reduce the formation of polymers.
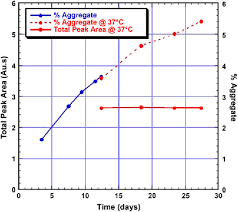
In the process of antibody production, the upstream usually determines the rate of formation of a polymer, and its environment such as temperature, protein concentration, pH, and ionic strength all influence the number of polymers. In a study on polymer, the SEC method was used to determine the content of the polymer. The experimental results are shown in Figure 2 as below. During D3-D13, the polymer content increased linearly with the increase of antibody concentration. At D13, the cells were removed from the medium and the harvested antibody-containing culture fluid was incubated at 37°C for two weeks. It was found that the content was still increasing slowly, even if the antibody concentration did not change. The longer the incubation time was, the higher the polymer content. This study shows that the polymer content can be reduced by reducing the culture time.
Figure 2 Changes in polymer content during cell culture (Image from reference 2)
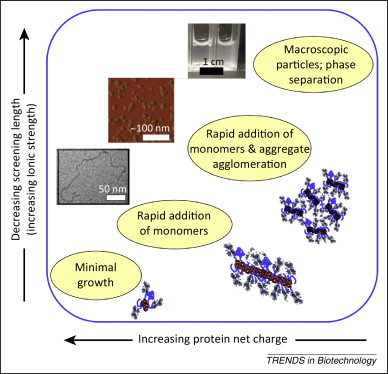
The solution environment of the antibody, adsorption with the container, and chemical degradation can all affect the concentration of the intermediates and their interactions. The rate of formation and content of the polymer can be reduced by changing the dissolution environment. For example, the adjustment of pH (adjusting pH to keep it away from the isoelectric point pI) and ionic strength can increase the difficulty of polymer growth, as shown in Figure 3. The adjustment of pH and ionic strength can change the charge that the antibody carries, so that the electrostatic repulsive force between the monomers is increased to counteract the strong hydrophobic interaction force between the “hot spots” and inhibit the formation of the polymer. It is generally believed that the effect of negative charge increase is more significant than positive charge. This study has been applied to a variety of polymer systems, such as monoclonal antibodies, cytokines, and globulins.
Fig 3 Effect of antibody charge on polymer growth (Source: reference 2)
In addition, disulfide bonds are also considered to be a factor for the formation of polymers (disulfide bonds can increase β-sheets), and the normal attachment of disulfide bonds is one of the conditions for the normal folding of proteins. The thiol group is an important source of disulfide bonds. The free thiol group on the cysteine that is not bound will result in a decrease in the disulfide bond content, which in turn will cause the protein to misfold. The formation of disulfide bonds requires a certain oxidizing environment, so the addition of oxidant Gu2+ promotes the formation of disulfide bonds. In addition, free thiol groups will affect the long-term stability of the antibody, and the removal of free thiol groups can increase the stability of the antibody.
For the upstream uncontrollable polymer, the downstream will take the removal role of the polymer. Variety of buffers being used in the purification process is an important factor affecting the polymer content. The acid buffer used to elute the antibody from protein A will cause the formation of polymers. Studies have found that the addition of arginine to the buffer in this step will reduce the amount of the solution compared to citrate, glycine and histidine. Multi-screen SEC column analysis can remove the polymer based on the size of the monomer and the polymer, but sacrifices some of the recovery. The mechanism of AEX and CEX to reduce the rate of the polymer is mainly that it can decompose the dimer or the multimer into monomers, and the content of the polymer under optimal conditions can be reduced to less than 0.5%. Frequent filtration and buffer exchange in the UF/DF stage, the mechanical pressure may cause the increase of protein polymers. However, as with the SEC principle, ultrafiltration can also be used in polymer removal.
The control of antibody polymers can be achieved not only during production and storage, but also during initial drug development. For example, a. experiments performed under high temperature conditions screen for proteins that are least likely to be misfolded and polymer; b. looking for proteins of different origins that are similar to the target protein (naturally can inhibit the formation of polymers), and compare the structure and sequence of the two proteins, and screen out proteins that are not easily aggregated.
References:
1.Therapeutic Protein Aggregation: Mechanisms, Design, and Control
2.Protein Aggregation and Bioprocessing
3.When GoodGoes Awry: The Aggregation of Protein Therapeutics