Triple-negative breast cancer accounts for approximately 15% of all breast cancer cases. Patients with this subtype typically have a poorer prognosis compared with other breast cancers, suggesting the need for improved existing treatments. A new treatment being studied at Moffitt Cancer Center involves an oncolytic virus that infects and kills cancer cells. In a recent clinical study, Dr. Hatem Soliman and his research team at Moffitt Cancer Center share the results of a phase 2 clinical trial of a combination of the oncolytic virus talimogene laherparepvec (TVEC) and standard chemotherapy for patients with early-stage triple-negative breast cancer. The results of the study were published online on Feb. 9, 2023, in Nature Medicine in a paper titled “Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial”.
Patients with triple-negative breast cancer lack expression of estrogen receptors and progesterone receptors, and have little to no expression of human epidermal growth factor receptor 2 (HER2) protein. Therefore, hormone therapy and drugs targeting HER2 are not effective in triple-negative breast cancer. The standard therapy for early-stage triple-negative breast cancer was chemotherapy, with the recent addition of the immune checkpoint inhibitor pembrolizumab. However, this approach has been associated with significant side effects. Many studies have shown that patients with higher levels of immune cells within the tumor tend to respond better to treatment. These observations suggest that drugs that stimulate the immune system may be beneficial in triple-negative breast cancer.
TVEC is a modified herpes simplex 1 virus that contains the sequence encoding the GM-CSF protein that stimulates the immune system. It is injected directly into the tumor and replicates within the tumor cells, leading to tumor cell breakdown and production of tumor-derived antigens. Immune cells recognize these antigens, infiltrate the tumor, and target the cancer cells to cause destruction. In addition, GM-CSF expressed by this virus acts as a beacon to help recruit immune cells to the tumor.
TVEC is approved for the treatment of advanced melanoma. In this new study, these authors wanted to evaluate whether a combination of oncolytic virus and standard chemotherapy in patients with triple-negative breast cancer before surgery could also be effective. In a phase 2 clinical trial of 37 patients, 45.9% responded, 89% remained disease-free two years after treatment, and those who developed a strong response did not relapse. The safety profile was not significantly different from that expected with standard chemotherapy, except for higher levels of low-grade fever, chills, headache, and injection site pain.
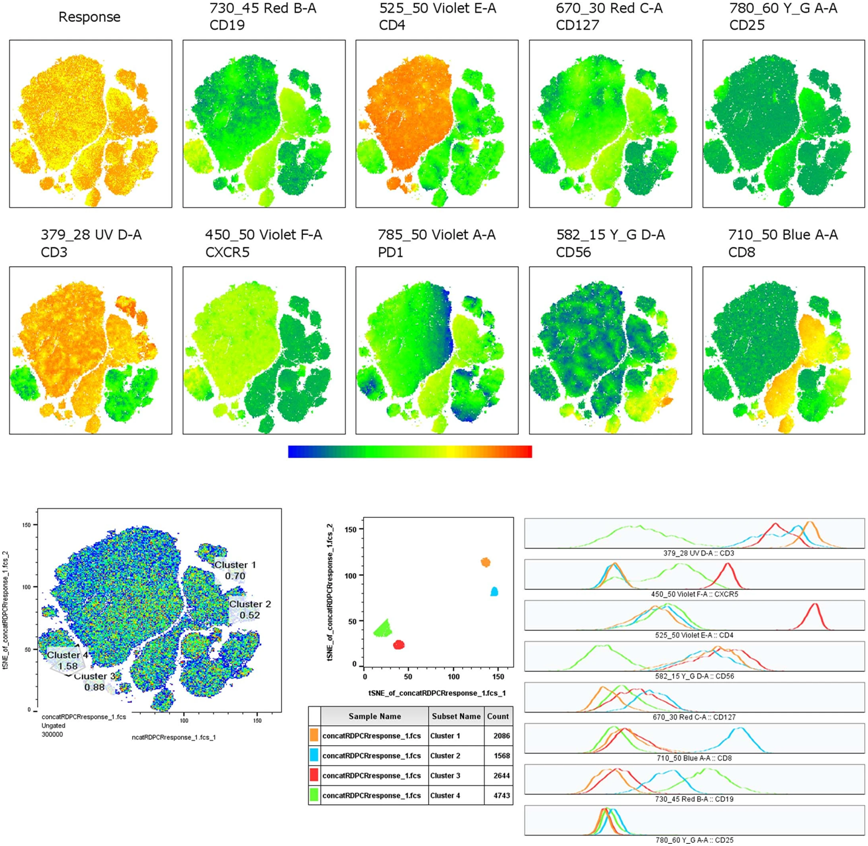
These authors also analyzed the levels of immune biomarkers and assessed whether these biomarkers correlated with patients’ response to treatment. They found that most tumor samples had higher levels of activation of anti-tumor T cells and immune signaling pathways during the first six weeks of treatment. Patients who showed a better response to treatment had higher levels of CD8 T cells at week six than those who had a poor response to treatment. These observations suggest that early activation of the immune response may lead to a better prognosis for patients with triple-negative breast cancer.
Soliman said that the results suggest that TVEC, when combined with systemic chemotherapy, may increase the response in high-risk, early-stage triple-negative breast cancer. Evidence of robust immune activation within the tumor warrants further study of TVEC in combination with current chemoimmunotherapy for the treatment of triple-negative breast cancer.
Reference
1. Soliman, Hatem, et al. “Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: A phase 2 trial.” Nature Medicine 29.2 (2023): 450-457.
