The single-domain antibody has similar antigen binding characteristics to the traditional antibody. But, because single-domain antibodies use a single Ig variable domain for antigen recognition, they can access epitopes that cannot be reached by conventional antibodies or antibody derivatives (e.g., scFv), such as penetrating protein surfaces or gaps in domain-domain media. In some cases, single-domain antibodies can cross-react with homologous targets from other species, which may contribute to the transition from preclinical application to clinical application. For example, anti-EGFR single-domain antibodies 8B6, anti-HER2 single-domain antibodies 2Rs15d, and single-domain antibodies against fibronectin EIIIB splicing variants cross-react with human and mouse antigens.
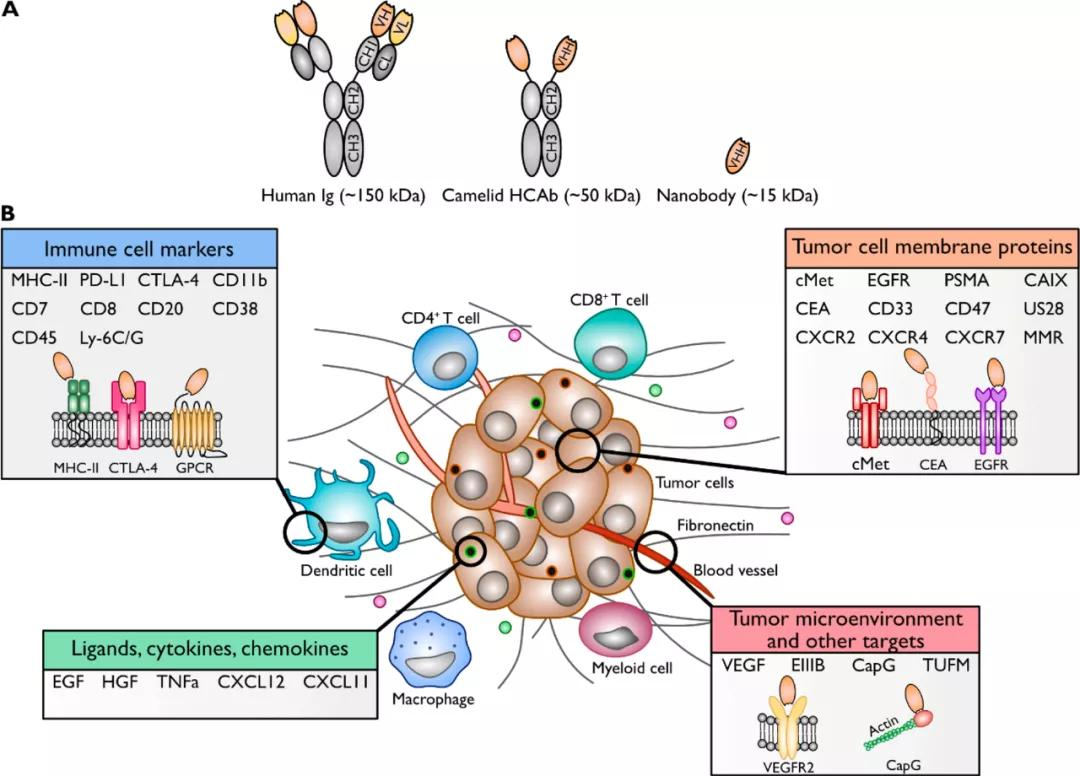
Fig.1 Single-domain antibodies and their targets associated with the tumor microenvironment
- The EGFR Family
Members of the epidermal growth factor receptor (EGFR) family are usually overexpressed on the surface of epithelial-derived tumor cells and play a role in their proliferation, survival, and angiogenesis. Antibodies targeting EGF receptors have been proved to be successful in cancer treatment. For example, the commercial product cetuximab is a human-mouse chimeric monoclonal antibody that specifically binds to EGFR. Therefore, members of the EGFR family have become one of the first tumor markers targeted by single-domain antibodies. The single-domain antibodies targeting EGFR1 were identified by phage display, and specific conjugates were identified by competitive elution with ligand EGF. Using the same EGFR phage single-domain antibody library and selecting EGFR extracellular domain, the researchers identified single-domain antibodies 7C12, 7D12, and 9G8, in which 7C12 and 7D12 competed with cetuximab.
Multivalent single-domain antibody molecules can be constructed by fusing single-domain antibody gene fragments or by chemical coupling. EGFR-specific single-domain antibodies form bivalent molecules in different combinations, all of which inhibit the proliferation of tumor cells in an epidermoid carcinoma model in vitro. Specifically, the combination of 7D12-9G8 anti-EGFR single-domain antibody performed best in inhibiting EGFR signal transduction and reducing the growth of human epidermoid carcinoma A431 cells. When bound to serum albumin binding single-domain antibody Alb1, this structure is called CONAN-1, which strongly inhibits EGF-induced signal transduction and leads to tumor regression in A431 xenografted mice.
Using similar methods, other researchers have obtained anti-EGFR single-domain antibodies 8B6 and OA-cb6. In addition, single-domain antibodies that recognize HER2, another member of the EGFR family, have been shown to specifically target tumors derived from HER2+SKOV3 ovarian cancer cells. Single-domain antibodies 11A4 and 5F7GGC targeting HER2 have been used in various clinical applications.
- VEGFR2 and VEGF
Vascular epithelial growth factor receptor 2 (VEGFR2) is a part of the human VEGFR receptor family, which exists in vascular endothelial cells. Its ligand VEGF is secreted by cell types such as macrophages and tumor cells, inducing downstream signaling pathways to participate in cell proliferation, angiogenesis, and metastasis, which makes VEGF and VEGFR2 attractive targets for single-domain antibody-based therapies. For example, VEGF (VEGFR) target antibodies can specifically bind to VEGF (VEGFR) to inhibit downstream signal pathways and angiogenesis.
Anti-VEGFR2 single-domain antibody 3VGR19 was obtained by phage display on the recombinant extracellular domain of the VEGFR2 receptor. It can inhibit VEGFR2 signal transduction and thus inhibit the formation of capillary-like structure, which has been confirmed in an in vitro study of human umbilical vein endothelial cells (HUVEC).
Some researchers isolated anti-angiogenic VEGFR2-D3 specific single-domain antibody NTV1 from HuSdl™. HuSdl™ is a human single-domain antibody library of “camelid” human antibodies. In a similar manner, VEGF single-domain antibodies were also obtained. These single-domain antibodies inhibited endothelial cell proliferation in vitro angiogenesis experiments using HUVEC. One of the humanized versions of Nb42 has also been produced. In addition, the single-domain antibody VA12, which specifically targets the VEGF-A binding domain, has shown the potential for anti-angiogenesis in chorioallantoic membrane assay.
- C-Met and HGF
Hepatocyte growth factor (HGF) binding to c-Met receptors can activate pathways that lead to cancer progression, angiogenesis, and metastasis. The overexpression of HGF and c-Met receptors is associated with a poor prognosis of several different epithelial and non-epithelial cancers. Currently, single-domain antibodies targeting c-Met and HGF have been produced. Schmidt Sl ø rdahl et al. used a bispecific single-domain antibody, one of which targets c-Met and the other that binds to human serum albumin to prolong the half-life. This bispecific anti-c-Met single-domain antibody inhibits the interaction between c-Met and HGF and leads to a decrease of cell migration and adhesion in multiple myeloma cells. This bispecific single-domain antibody is more effective than the traditional bivalent anti-c-Met monoclonal antibody in inhibiting tumor growth.
Jo Vercammen et al. reported that two α HGF- single-domain antibodies called 1E2 and 6E10 showed a dose-dependent inhibition of HGF-induced proliferation of Bx-PC3 human pancreatic cancer cells. Nude mice bearing human glioma U-87MG xenograft were treated with an anti-HGF single-domain antibody. Compared with the control group, the anti-HGF single-domain antibody significantly inhibited tumor growth. Both of the above single-domain antibodies show potential for the treatment of multiple myeloma and other HGF-c-Met-driven cancers.
- Other Targets
In addition to the above molecules, many other tumor-associated antigens have also been used as targets for the development of single-domain antibodies. Chemokine receptors, belonging to G protein-coupled receptors (GPCR), are overexpressed in many kinds of malignant tumors. Chemokines and their receptors drive the migration and activation of various cell types associated with innate and adaptive immune responses. If the goal is to interfere with cell migration, given the excellent tissue permeability of single-domain antibodies, these molecules will be ideal targets. Single-domain antibodies targeting GPCR and its ligands include reagents against human CXCR2, CXCR4, CXCR7, CXCL11, and CXCL12, as well as viral GPCRUS28.
In addition, single-domain antibodies targeting human tumor-associated transmembrane/membrane proteins have been identified, such as carcinoembryonic antigen (CEA), prostate-specific membrane antigen (PSMA), and human and mouse macrophage mannose receptor (MMR).
Immune cell markers are also important targets being investigated, such as human CD7, human and mouse CTLA-4, human and mouse PDL-1, mouse CD8, mouse CD11b, human CD20, human CD38, mouse CD45, mouse Ly-6C/Ly-6G, and human and mouse MHC-II. Other targets include fibronectin, TUFM, CapG, CAIX, CD33, human and mouse CD47, mouse ARTC2, and TNF α.
