Next-IO™ 4-1BB × PD-L1 Therapeutic Bispecific Antibody Program
About This Program
This program aims to develop 4-1BB × PD-L1 therapeutic bispecific antibody for cancer immunotherapy.
Rationale for our program:
-
Blocking of the PD-1 / L1 axis has shown durable responses and prolonged overall survival in a variety of cancer types. However, there is still a significant unmet need for relapsed patients.
-
Activation of the tumor necrosis factor receptor (TNFR) superfamily reflects the Next-IO™ cancer immunotherapy. 4-1BB-mediated co-stimulation directs the fate of antigen-stimulated T and NK cells and leads to cell activation, survival, and spread.
-
Current agonist treatment interventions for 4-1BB have shown great promise for cancer immunotherapy.
Here, we propose a novel combination: 4-1BB × PD-L1 therapeutic bispecific antibody, which may result in an increased therapeutic efficacy by specifically activating 4-1BB expressing cells in the tumor niche where PD-L1 is simultaneously expressed.
4-1BB × PD-L1 in Cancer Studies
Binding of PD-L1 to PD-1 on activated T cells inhibits expansion and survival of CD8-positive T cells, suppresses the immune system and leads to immune evasion. Blocking PD-1 abolishes T cell inhibition, activates antigen-specific T lymphocytes and enhances cytotoxic T cell-mediated tumor cell lysis, which may result in reduced tumor growth.
4-1BB (Tumor Necrosis Factor Receptor Superfamily Member 9; TNFRSF9) is a surface glycoprotein of the tumor necrosis factor receptor superfamily. As an inducible costimulatory receptor, 4-1BB plays a key role in T cell proliferation, survival, and cytolysis.
Colorectal Cancer
-
Colorectal cancer is one of the tumors with poor efficacy in immunotherapy. The vast majority of patients with MMR or microsatellite stabilization (MSS) do not benefit from immunotherapy.
-
There is a need to develop predictive biomarkers and effective therapeutic combination strategies.
-
To date, NO ongoing clinical trials have focused on the development of BiAbs for targeted treatment of colon cancer. Our program will be a pioneer.
Ongoing Clinical Trials
-
Currently, as far as we know, NO bispecific anti-4-1BB × PD-L1 antibody is undergoing a clinical trial. But accumulated preclinical data are emerging, clarifying the synergistic anti-tumor effects of this bispecific combination.
-
We believe that this dual targeting strategy will provide insights into the tumor immunotherapy, especially in the treatment of colorectal cancer. In an effort to optimally leverage 4-1BB × PD-L1-mediated immune response, our Next-IO™ 4-1BB × PD-L1 targeted antibody program attempts to explore the optimal combination strategy - that is, how to exert the best anti-tumor outcome while synergistically produce CD47 × PD-L1.
Program Plan
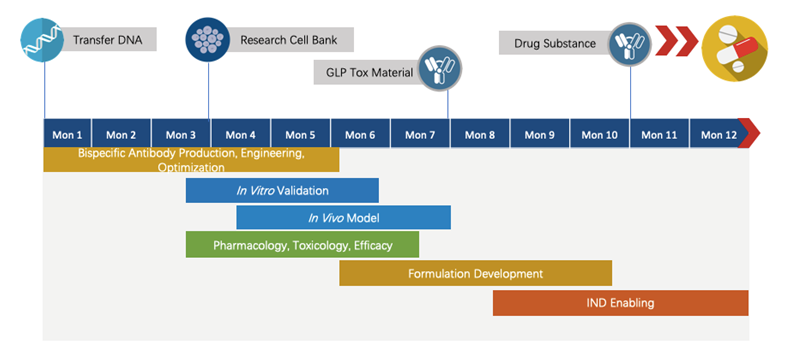
We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop 4-1BB × PD-L1 therapeutic bispecific antibody program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership together. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners further their programs with more chances to succeed. Look forward to cooperating with you in the near future.
For Research Use Only | Not For Clinical Use



 Download our brochure
Download our brochure