Next-IO™ Anti-PD1 × TIM3 Bispecific Antibody Program
As a thriving CRO company, Creative Biolabs has initiatives to acquire, develop and commercialize Next-IO™ programs for the therapeutic purpose. Our anti-PD1 × TIM3 bispecific antibody program aims to develop bispecific antibodies that could recognize and potentially, against programmed death 1 (PD1), T cell immunoglobulin, and mucin-domain containing-3 (TIM3).
Background
The immune activities happening inside the immune system are critical when researching on cancer therapy. Among all the immune activities, immune suppression may be the obstacle. However, studies have demonstrated PD1 works as an immune-suppressing marker, and block PD1/PDL1 interaction can partially restore T cell function. Several anti-PD1 monoclonal antibodies have been approved by officials because they have great clinical anti-tumor outcomes. Among these, TIM3 is an immune checkpoint expressed on tumor infiltrating lymphocytes (TILs) and blockade of TIM3 has shown promising efficacy in several ongoing clinical trials. The combination of PD1, TIM3, and TILs exhibit the most deleterious phenotype - may fail to proliferate and produce IL-2, TNF, and IFN-γ. But, studies indicated combined targeting of PD1 and TIM3 is most effective to control tumor growth, in comparison to targeting either pathway alone.
There are two ways to achieve combined-targeting of PD1 and TIM3. One is to develop anti-PD1 and TIM3 bispecific antibody. The bispecific antibody comprises an antigen-binding site that specifically binds to PD1 and another site binds to TIM3. Another way is making an anti-TIM3 monoclonal antibody with an anti-PD1 monoclonal antibody. Our program will focus on the bispecific antibody drug discovery and development.
Published Data
-
TIM3 and PD1 co-expressing CD8 positive TILs comprise a major population (44.9%) of CD8 T cell present in TILs infiltrating various solid tumors.
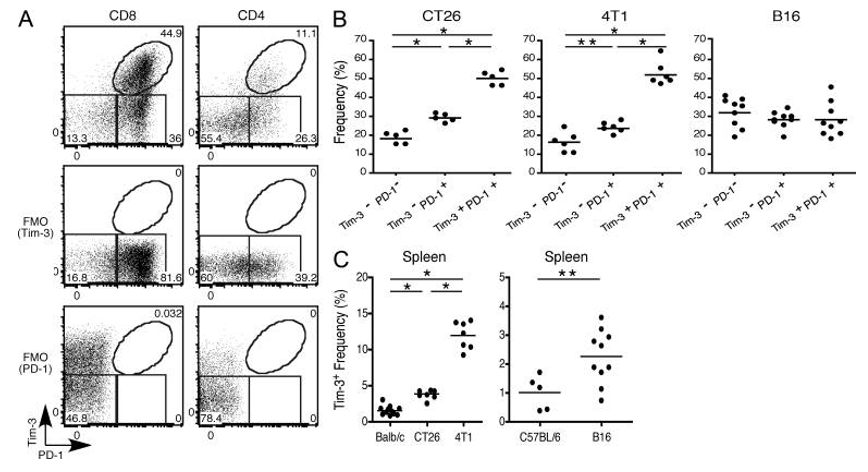 Fig.1 TIM3 and PD1 co-expressing CD8 positive TILs comprise a major population (44.9%).1
Fig.1 TIM3 and PD1 co-expressing CD8 positive TILs comprise a major population (44.9%).1
-
TIM3 and PD1 co-expressing CD8 positive TILs exhibit exhausted phenotype - impaired IL-2, TNF, IFN-γ production, and restricted proliferation. This suggests co-expression of TIM3 and PD1 marks the most exhausted population of TILs in the tumor microenvironment.
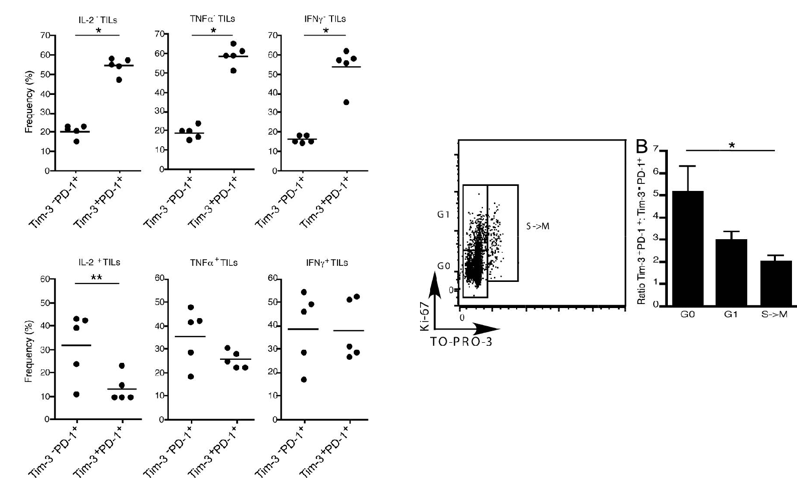 Fig.2 TIM3 and PD1 co-expressing CD8 positive TILs exhibit exhausted phenotype - impaired IL-2, TNF, IFN-γ production, and restricted proliferation.1
Fig.2 TIM3 and PD1 co-expressing CD8 positive TILs exhibit exhausted phenotype - impaired IL-2, TNF, IFN-γ production, and restricted proliferation.1
-
Blockade of TIM3 and PD1could restore T cell immune functions and inhibit tumor growth.
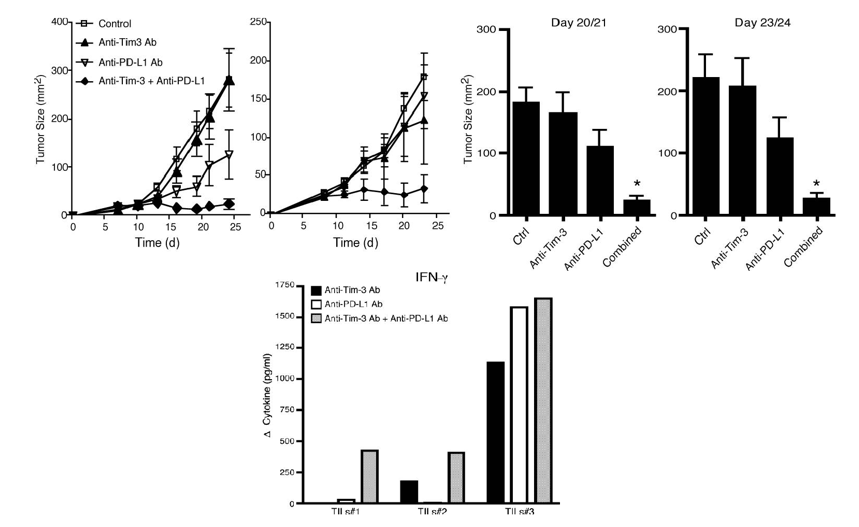 Fig.3 Blockade of TIM3 and PD1could restore T cell immune functions and inhibit tumor growth.1
Fig.3 Blockade of TIM3 and PD1could restore T cell immune functions and inhibit tumor growth.1
-
Combined treatment of anti-TIM3 and anti-PDL1 exhibit an anti-tumor effect in acute myeloid leukemia (AML) models.
From these data, we learn that combine therapy of anti-TIM3 and anti-PD1 can result in a better survival benefit, compared with single pathway treatment. The dual blockade of anti-TIM3 and ant-PD1 can improve proliferation and cytokine production in vitro. And in a study using animal models, it has been demonstrated that this dual-blockade can improve INFγ production and restrict tumor growth. In summary, we believe bispecific anti-TIM3/PD1 will show great anti-tumor effects.
Ongoing Clinical Trials
So far, monoclonal antibodies developed against programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) have all shown some level of anti-tumor efficacy, and several antibodies have already approved by officials to use in practice. TIM3, as a key immune checkpoint, is currently being evaluated in many studies and clinical trials. Till today, not one drug is approved. We believe our program of discovering and researching on bispecific TIM3/PD1 is at the cutting edge of the field and have certain marking and sale potential.
Program Planning and Management
We have extensive experience in performing comprehensive program developments and effective problem-solving. For our Next-IO™ programs, we are committed to delivering the finalized program to our partners within about 1.5 years. The accurate timeline will be determined on a case-by-case basis. Here is a draft timeline for your glance.
 Fig.4 The timeline of Next-IOᵀᴹ programs.
Fig.4 The timeline of Next-IOᵀᴹ programs.
Collaboration
Creative Biolabs is looking for partners to help us to gain the competitive edge in the field and maximize the impacts of the programs. With your support and collaboration, we are dedicated to developing novel cancer immunotherapies that can hope bother parties to achieve a greater success.
If you are interested in our programs, please feel free to contact us for more details.
References
-
Sakuishi, Kaori, et al. "Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity." Journal of Experimental Medicine 207.10 (2010): 2187-2194.
-
Fourcade, Julien, et al. "Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients." Journal of Experimental Medicine 207.10 (2010): 2175-2186.
For Research Use Only | Not For Clinical Use


 Fig.1 TIM3 and PD1 co-expressing CD8 positive TILs comprise a major population (44.9%).1
Fig.1 TIM3 and PD1 co-expressing CD8 positive TILs comprise a major population (44.9%).1
 Fig.2 TIM3 and PD1 co-expressing CD8 positive TILs exhibit exhausted phenotype - impaired IL-2, TNF, IFN-γ production, and restricted proliferation.1
Fig.2 TIM3 and PD1 co-expressing CD8 positive TILs exhibit exhausted phenotype - impaired IL-2, TNF, IFN-γ production, and restricted proliferation.1
 Fig.3 Blockade of TIM3 and PD1could restore T cell immune functions and inhibit tumor growth.1
Fig.3 Blockade of TIM3 and PD1could restore T cell immune functions and inhibit tumor growth.1
 Fig.4 The timeline of Next-IOᵀᴹ programs.
Fig.4 The timeline of Next-IOᵀᴹ programs.
 Download our brochure
Download our brochure