Next-IO™ Anti-CSF1/CSF1R Axis Targeted Therapeutic Antibody Program
About This Program
This program aims to develop anti-CSF1/CSF1R axis targeted therapeutic antibody for immuno-oncology.
Tumor-associated macrophages (TAM) often account for a large proportion of all tumor-infiltrating immune cells and exert complex immune functions in the tumor microenvironment. Colony-stimulating factor 1 (CSF1) controls the proliferation, differentiation, and survival of macrophages from their precursors. CSF1 receptor (CSF1R) signaling in TAM promotes its immunosuppressive and pro-inflammatory M2-like phenotype.
Studies have shown that expression of CSF1 is associated with poor prognosis in reproductive system tumors such as ovarian, uterine, breast and prostate cancers. Preclinical studies have shown that CSF1R blockade can delay tumor growth in some mouse cancer models by elimination or repolarization of TAM. In addition, data have shown that compensation between CSF1R+ macrophages and Foxp3+ Treg cells drives resistance to tumor immunotherapy, which provides a theoretical basis for the design of combination immunotherapy.
CSF1/CSF1R Axis
CSF1R is a tyrosine kinase receptor that spans the cell membrane and is activated by binding of two known cytokine ligands: CSF1 and IL-34. After ligand binding, the two receptor molecules are paired and several key tyrosine residues that protrude into the receptor portion of the cell are phosphorylated. This acts as a binding platform for downstream signaling molecules and activates several signaling cascades, including PI3K / AKT, mitogen-activated protein kinase, and SRC pathways. Multiple studies have shown that:
-
These pathways ultimately maintain the growth, proliferation, survival, differentiation, and function of macrophages and other bone marrow cells, including bone marrow-derived suppressor cells.
-
The CSF1R pathway may also play an important role in macrophage polarization; i.e, M1 / M2 dichotomy, which is of great significance for the development of various pathological conditions.
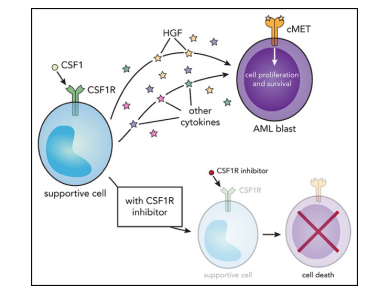 Fig.1 CSF1R inhibitors exhibit antitumor activity in AML.4
Fig.1 CSF1R inhibitors exhibit antitumor activity in AML.4
CSF1/CSF1R Axis in Cancer Studies
The targeting of the CSF1/CSF1R axis-targeted / Fas system in therapeutic strategies is particularly complex, due to the intricate signal pathway interaction with other molecules. Here are some published data about CSF1/CSF1R work as a potential target for cancer immunotherapy.
-
Tumor cell-derived CSF1 promotes tumor growth.
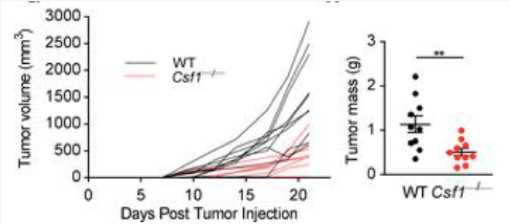 Fig.2 In vivo growth curves and tumor masses of WT and Csf1–/– MC38 tumor cells in WT mice following s.c. injections at day 0.1
Fig.2 In vivo growth curves and tumor masses of WT and Csf1–/– MC38 tumor cells in WT mice following s.c. injections at day 0.1
-
Combined inhibition of CSF1R and PI3Kδ effectively blocks tumor immunosuppression.
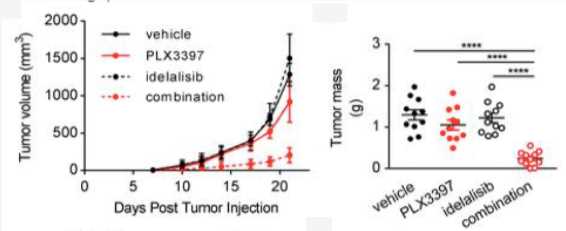 Fig.3 In vivo growth curves and tumor masses at day 21 of WT mice orally treated with vehicle or PLX3397 or idelalisib or the combination of the 2 from day 7 following s.c. injections of MC38 cells.1
Fig.3 In vivo growth curves and tumor masses at day 21 of WT mice orally treated with vehicle or PLX3397 or idelalisib or the combination of the 2 from day 7 following s.c. injections of MC38 cells.1
-
CSF1R blockade enhances the therapeutic efficacy of anti-PD1 treatment in melanoma models.
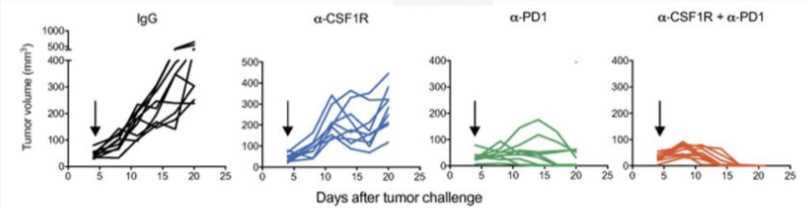 Fig.4 Tumor volumes of subcutaneous SM1-OVA melanomas treated as indicated. IgG, α-CSF1R, α-PD1, and α-CSF1R + α-PD1.2
Fig.4 Tumor volumes of subcutaneous SM1-OVA melanomas treated as indicated. IgG, α-CSF1R, α-PD1, and α-CSF1R + α-PD1.2
Ongoing Clinical Trials
-
Currently, several anti- CSF1/CSF1R antibodies are currently being evaluated in clinical trials after successful testing in animal models. An increasing number of therapeutic molecules are getting confirmed on its role in immune responses and early clinical trial data showed great promise. However, further studies are needed to optimize the efficacy, safety, and combination strategies to achieve greater clinical success.
-
In this case, CSF1/CSF1R axis is still a compelling target for cancer immunotherapy. In an effort to optimally leverage CSF1/CSF1R axis-mediated immune response, the future of anticancer strategies aimed at CSF1R most likely lies in combinations that include immune checkpoint therapies and chemotherapy.
Table 1. Ongoing clinical trials of anti-CSF1/CSF1R antibodies
|
Target
|
Compound
|
Clinical Phase
|
Sponser
|
Indication
|
ClinicalTrials.gov identifier
|
Status/Results
|
|
CSF1R
|
Emactuzumab (RG7155)
|
1
|
Roche
|
Solid tumors
|
NCT01494688
|
PMR: 5/44 (11%)
ORR: 0%
CBR: 6/40 (24%)
|
|
CSF1R
|
AMG820
|
1
|
Amgen
|
Solid tumors
|
NCT01444404
|
ORR: 1/25 (4%)
CBR: 6/25 (24%)
|
|
CSF1R
|
IMC-CS4 (LY3022855)
|
1
|
Eli Lilly
|
Solid tumors
|
NCT01346358
|
Ongoing
|
|
1
|
Eli Lilly
|
Breast and prostate cancer
|
NCT02265536
|
Ongoing
|
|
CSF1
|
MCS110
|
1/2
|
Novartis
|
Prostate cancer
|
NCT00757757
|
Terminated
|
Program Plan
We have extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years prior to entering the IND stage.

Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop anti-CSF1/CSF1R axis targeted therapeutic antibody program together. Our scientists are dedicated to bringing together years of valuable experience to our partner and achieve a meaningful partnership. For commercial partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners and their programs enter the market faster. Look forward to cooperating with you in the near future.
References
-
Gyori, D.; et al. Compensation between CSF1R+ macrophages and Foxp3+ Treg cells drives resistance to tumor immunotherapy. Jci Insight, 2018, 3(11).
-
Neubert, N J.; et al. T cell-induced CSF1 promotes melanoma resistance to PD1 blockade. Science Translational Medicine, 2018, 10(436).
-
Cannarile, M A.; et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. Journal for Immunotherapy of Cancer, 2017, 5(1): 53.
-
Edwards, D K.; et al. CSF1R inhibitors exhibit antitumor activity in acute myeloid leukemia by blocking paracrine signals from support cells. Blood, The Journal of the American Society of Hematology, 2019, 133(6): 588-599.
For Research Use Only | Not For Clinical Use


 Fig.1 CSF1R inhibitors exhibit antitumor activity in AML.4
Fig.1 CSF1R inhibitors exhibit antitumor activity in AML.4
 Fig.2 In vivo growth curves and tumor masses of WT and Csf1–/– MC38 tumor cells in WT mice following s.c. injections at day 0.1
Fig.2 In vivo growth curves and tumor masses of WT and Csf1–/– MC38 tumor cells in WT mice following s.c. injections at day 0.1
 Fig.3 In vivo growth curves and tumor masses at day 21 of WT mice orally treated with vehicle or PLX3397 or idelalisib or the combination of the 2 from day 7 following s.c. injections of MC38 cells.1
Fig.3 In vivo growth curves and tumor masses at day 21 of WT mice orally treated with vehicle or PLX3397 or idelalisib or the combination of the 2 from day 7 following s.c. injections of MC38 cells.1
 Fig.4 Tumor volumes of subcutaneous SM1-OVA melanomas treated as indicated. IgG, α-CSF1R, α-PD1, and α-CSF1R + α-PD1.2
Fig.4 Tumor volumes of subcutaneous SM1-OVA melanomas treated as indicated. IgG, α-CSF1R, α-PD1, and α-CSF1R + α-PD1.2

 Download our brochure
Download our brochure