Next-IO™ CD47 × MSLN Therapeutic Bispecific Antibody Program
About This Program 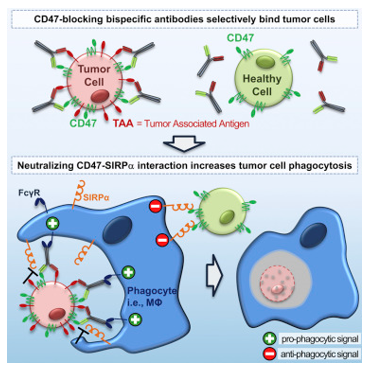
This program aims to develop CD47 × MSLN therapeutic bispecific antibody for immuno-oncology.
Mesothelin (MSLN) is a cell surface glycoprotein widely expressed in solid tumors. It is believed to associate with poor prognosis of many cancers. Monoclonal antibodies (mAbs) against MSLN have shown limited clinical efficacy in this case.
CD47 is a checkpoint in innate immune cells that provides a "don't eat me" signal to allow healthy cells to escape phagocytosis. Similar to MSLN, a high level of CD47 expression associates with poor prognosis, and due to the prevalence of CD47 in the immune system, the ability of mAbs alone to block CD47 is hampered.
Hence, we are hoping to develop a bispecific antibody (biAb) with (i) high-affinity targeting arm (anti-mesothelin) to inhibit tumor growth, and (ii) an effector arm (anti-CD47) arm to prevent TAA binding to non-cancerous cells.
CD47 × MSLN
MSLN is a lineage-restricted cell surface protein expressed at a very low level in mesothelial cells of healthy tissues. It is also a tumor differentiation antigen that produced in a wide range of solid tumors, including mesothelioma, pancreas, biliary tract, ovarian cancer, lung cancer, and gastric cancer.
CD47 is a common membrane protein found in almost all cell types. Many cancers are shown to be over-activated by the CD47 signal, aka "Don't Eat Me" signal. It can evoke immune interaction with the cellular signal receptor protein alpha (SIRPα) to prevent programmed cell removal (PCR). In particular, binding of SIRPα to CD47 promotes tyrosine phosphorylation in the cytoplasmic region of SIRPα, further producing the down-regulated signal to inhibit phagocytosis.
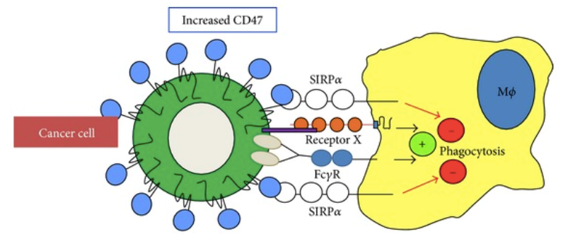 Fig.1 CD47 regulates phagocytosis of host cells by interacting with SIRPα. (Oldenborg, 2013)
Fig.1 CD47 regulates phagocytosis of host cells by interacting with SIRPα. (Oldenborg, 2013)
CD47 × MSLN in Cancer Studies
Here are some published data about CD47 × MSLN working as a potential target for cancer immunotherapy.
-
The combined effect by anti-CD47 / CD19 biAb has better ability to mediate phagocytosis than CD47 or CD19 alone.
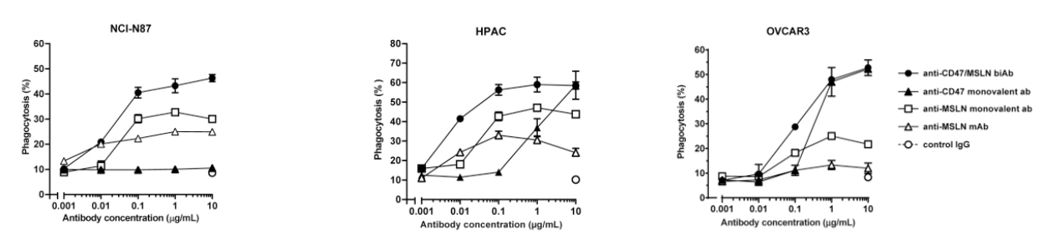 (Dheilly, 2017)
(Dheilly, 2017)
Ongoing Clinical Trials
-
Currently, only one anti-CD47 / MSLN bispecific antibody (named NI-1801) developed by Novimmune is known to involve in the clinical phase I trials. But we believe it is still a compelling bi-functional combination to research in cancer immunotherapy.
-
The dual-targeting strategy will provide further insights into tumor immunotherapy, which allows us to fight cancer more efficient. In an effort to optimally leverage CD47 × MSLN-mediated immune response, our next-generation CD47 × MSLN targeted antibody program attempts to explore the optimal combination strategy - that is, how to exert the best anti-tumor outcome while CD47 × MSLN is synergistically expressed.
Program Planning and Management
We have extensive knowledge if end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.

Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop CD47 × MSLN Dual-Targeting Fusion Protein program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership together. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners to further their programs with more chance to succeed. Look forward to cooperating with you in the near future.
References
-
Oldenborg, P.A. CD47: a cell surface glycoprotein which regulates multiple functions of hematopoietic cells in health and disease. International Scholarly Research Notices. 2013, 2013.
-
Dheilly, E.; et al. Selective blockade of the ubiquitous checkpoint receptor CD47 is enabled by dual-targeting bispecific antibodies. Molecular Therapy. 2017, 25(2): 523-533.
For Research Use Only | Not For Clinical Use



 Fig.1 CD47 regulates phagocytosis of host cells by interacting with SIRPα. (Oldenborg, 2013)
Fig.1 CD47 regulates phagocytosis of host cells by interacting with SIRPα. (Oldenborg, 2013)
 (Dheilly, 2017)
(Dheilly, 2017)

 Download our brochure
Download our brochure