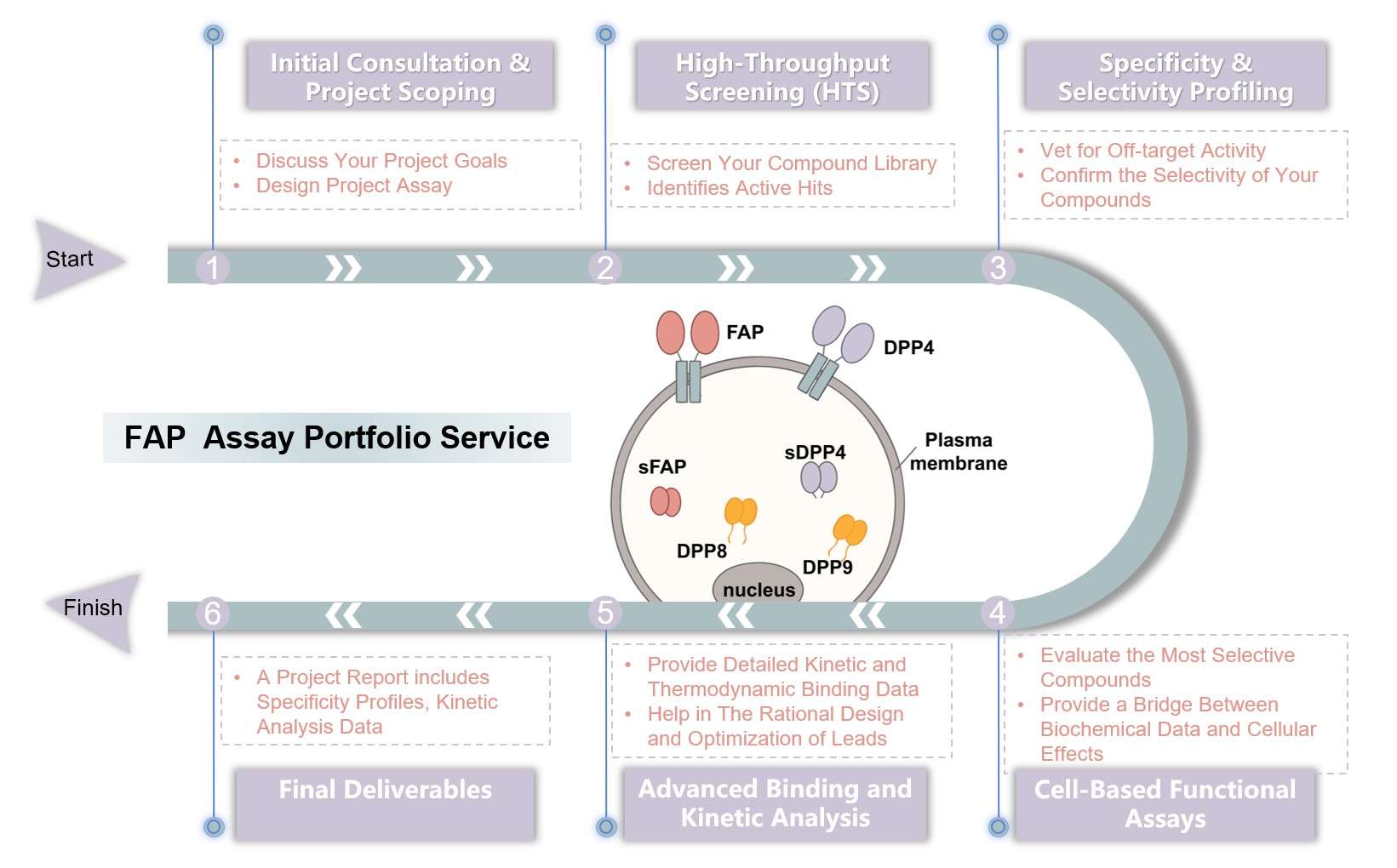
FAP Assay Portfolio Service
Introduction What We Can Offer Workflow Why Creative Biolabs Customer Reviews FAQs Related Services Contact Us
Creative Biolabs helps you accelerate drug discovery and confidently advance your lead compounds by providing comprehensive, validated screening and selectivity platforms. Our deliverables go beyond raw numbers, providing a complete profile of your compounds' activity, specificity, and binding kinetics, which are critical for de-risking your pipeline. Through advanced enzymatic, cell-based, and kinetic assays, we help you overcome cross-reactivity issues with homologous proteases like DPP4 and POP.
The Structure, Function, and Expression of FAP
FAP, a serine protease, is an attractive therapeutic target in oncology and fibrosis due to its high expression in disease states and minimal presence in healthy tissues. This unique expression profile makes it a compelling candidate for developing highly specific therapies that can target diseased cells while sparing healthy ones, thereby minimizing side effects. However, developing such therapies is complex, as FAP's structural similarity to other proteases, particularly Dipeptidyl Peptidase 4 (DPP4) and Prolyl Oligopeptidase (POP), presents a significant challenge.
Discover how we can help - Request a consultation.
What We Can Offer
|
Cellular and Mechanistic Analysis
|
Biochemical and Histological Readouts
|
-
Cell Viability and Apoptotic Assays
-
Colony Formation Assay
-
EMT Assays
|
-
ELISA and Immunoblotting Services
-
Hydroxyproline Assay
-
Masson Trichrome Staining
-
ISH, IHC, and Immunoprecipitation
|
Workflow of FAP Assay Portfolio Service
Highlights
Unmatched Expertise and Experience
Our team provides an unparalleled level of scientific insight. This expertise is a cornerstone of our service, ensuring that every project is guided by professional knowledge and a proven track record.
Rigorous, Multi-Stage Vetting
Our comprehensive, multi-stage workflow de-risks your pipeline by meticulously vetting compounds. This process acts as a scientific funnel, ensuring only the most promising candidates with confirmed on-target activity and minimal off-target effects are advanced.
High-Quality, Actionable Data
Our detailed reports provide clear, actionable insights that help you make confident decisions about which compounds to advance. Each report includes the key metrics and a comprehensive interpretation of the results, a proposed mechanism of action, and strategic recommendations.
A Proven Partner in Drug Discovery
By providing reliable and efficient services, we have helped numerous researchers and companies achieve significant milestones, including accelerated screening timelines and reduced off-target failures, as demonstrated by our published data and case studies.
Experience the Creative Biolabs advantage - Get a quote today.
Customer Reviews
-
Exceptional Specificity
Creative Biolabs' service improved our confidence in lead compound specificity. The counter-screening against homologous proteases like DPP4 and POP provided essential data, saving us time and resources. - Da*l.
-
Accelerated Screening
The high-throughput screening platform from Creative Biolabs greatly facilitated our compound library screening. We were able to screen thousands of compounds and identify strong hits in a fraction of the time it would have taken us to develop the assay in-house. - M*e K.
FAQs
What types of compounds can be screened using your service?
Our platform is highly flexible and can accommodate a wide range of compounds, including small molecules, biologics, and natural products. Our HTS protocols are optimized for diverse compound libraries, and we're happy to discuss your specific compound's properties during our initial consultation.
Can you help with lead optimization after the initial screening?
Absolutely. Our service is designed to support the entire drug discovery pipeline. Our advanced binding and kinetic analysis provide the mechanistic insights needed for rational lead optimization, and our team of scientists can help you interpret the data to guide your next steps.
Related Services
Cell Line Development Service
We can develop stable cell lines that robustly express FAP, which are essential for our cell-based functional assays. These customized cell lines are critical for providing a more biologically relevant context for your compound testing.
Learn More →
Protein Production
We offer custom services for expressing and purifying high-quality recombinant FAP. Our expertise ensures that the protein is produced with high purity and biological activity, which is essential for obtaining reliable and consistent results in your FAP assays.
Learn More →
How to Contact Us
Ready to advance your precision oncology initiatives? Creative Biolabs is your trusted partner in accelerating FAP-targeted drug discovery. Our expert-led FAP assay portfolio service is designed to provide the specific, reliable data you need to advance your projects with confidence.
Contact us for more information and to discuss your project.
For Research Use Only | Not For Clinical Use






