Function Assays for Immune Checkpoint
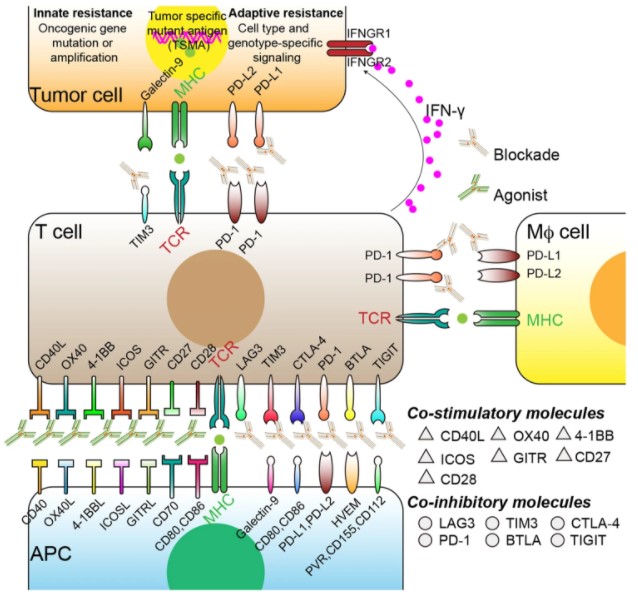
Several clinical trials have shown that immunotherapy targeting immune checkpoint molecules is a promising strategy for cancer treatment. By regulating the co-inhibitory pathways, the inhibition of T cell activation by tumor cells can be overcome, thus increasing the anticancer activity of the immune system. Despite the great success of immunotherapy targeting immune checkpoints, there is still much work to be done in research and development projects.
Taking advantage of advanced technology platforms and deep scientific and regulatory understanding in immune-oncology, Creative Biolabs has developed a novel one-stop service covering a full range of immune checkpoint functional assays for our global clients to discover and identify target molecules’ potential to modulate immune function.
Based on the unrivaled expertise and experiences in immunotherapy discovery and development, we can provide the following immune checkpoint function assay for our clients, but not limited to:
Custom T Cell activation and Proliferation Assay
Activation of T cells is mediated by the involvement of the TCR and CD3 complexes, followed by costimulatory molecules, such as the CD28 receptor. Stimulated by these two signals together, a series of activations take place inside the cell, including the release of cytokines and the proliferation of T cells to fight infection or disease.
By several approaches, like bioluminescent methods or ELISA, we can directly analyze its ability to restore T-cell functions. Meanwhile, the T cell activation and proliferation process can also be evaluated via cell counting kit-8 (CCK-8), MTT, CFSE as well as flow cytometric analysis, etc.
Cytokine Release Assay
Identifying the potential for cytokine release is a critical aspect of immune checkpoint therapy safety assessment. We provide several types of cytokine release assays, which can be customized to meet the demands of your program.
T Cell Cytotoxicity Assay
To study cytotoxicity effects in immunotherapies, several in vitro cytotoxicity assays have been developed. These cell-based assays include colorimetric-based approaches such as MTT and lactate dehydrogenase (LDH) assays, fluorescent-based assays using Calcein-AM, luminescent-based assays measuring adenosine triphosphate (ATP) readouts with reagents like homogeneous assays using cell lines engineered to express firefly luciferase. Creative Biolabs has robustly developed in vitro platforms to provide reproducible cell-based cytotoxicity assays for our clients all over the world.
Mixed Lymphocyte Reaction (MLR) Assay
What is more, measuring MLR is a useful method to understand the effects of biologics or small molecules on T cell activation, an important response in the tumor microenvironment (TME). The advantage of the MLR reaction is that the strong baseline response (reaction without added test compound) does not require stimulation with any other stimulatory compound. We can offer many different types of MLR assays, such as one-way, two-way, and three-way, to help global clients understand a drug candidate’s MOAs.
Creative Biolabs offers a cell-based checkpoint inhibitor assay service designed to evaluate immune checkpoint inhibitors in a biologically relevant environment. This service enables researchers to assess the effectiveness of inhibitors targeting key immune checkpoints like PD-1/PD-L1. By using custom cell lines and advanced assay techniques, Creative Biolabs provides detailed insights into immune cell activation, cytokine release, and tumor cell responses. This comprehensive service accelerates the discovery of novel immunotherapies, supporting the development of more effective cancer treatments.
Features of Our Service
-
Multiplexed detection
Multiple ways of secreted proteins to correlate communication via cytokines with cellular activity.
-
Biologically-related
Immune checkpoint functional assays are MOA-reflective, monitoring checkpoint signaling and testing of small molecule or biologic drugs.
-
Robust assays
Highly reproducible assays for potency for immunotherapy drug discovery and development.
Creative Biolabs is confident in providing immune checkpoint functional assays services of high quality to meet demands. As well, Creative Biolabs would like to provide many other immune-oncology services. If there is any query, please contact us for more details.
Reference
-
Ye C, Yang H, Cheng M, et al. A rapid, sensitive, and reproducible in vivo PBMC humanized murine model for determining therapeutic-related cytokine release syndrome. The FASEB Journal. 2020 Sep;34(9):12963-75.
For Research Use Only | Not For Clinical Use



 Download our brochure
Download our brochure