Next-IO™ GITR x CTLA-4 Therapeutic Bispecific Antibody Program
About This Program
This program aims to develop GITR x CTLA-4 therapeutic bispecific antibody for colorectal cancer immunotherapy.
Rationale for our program:
-
CTLA-4 is a checkpoint receptor that is highly expressed on tumor-infiltrating T cells, especially T regulatory cells (Tregs).
-
GITR is a member of TNFR superfamily, which is highly expressed on tumor-infiltrating T, especially Treg, but also includes NK cells and tumor cells.
-
Bispecific antibody (BsAb) is a novel antibody that mediates specific killing by targeting two different antigens and selectively redirecting effector cells to target cells. This enhanced synergistic anti-tumor effect highlights a promising approach to immunotherapy.
Our program combines the targeting of CTLA-4 and GITR simultaneously and expects to enhance Treg consumption and kill tumor cells through a variety of mechanisms, including consumption of Treg, activation of effector T cells, and activation of NK cells, therefore provide a promise for anti-tumor T cell immunity.
GITR x CTLA-4
GITR, also referred to as TNFRSF18, is a type I transmembrane protein from the tumor necrosis factor receptor superfamily. GITR is preferably expressed in natural killer (NK) and T cells, especially in Treg cells. Based on data from mouse tumor model studies, the therapeutic activity of GITR agonist has been associated with reduction and functional modulation of intra-tumor Treg cells, and strengthen anti-tumor CD8+ T cell functions.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4, CD152), an immune checkpoint, is a membrane glycoprotein involving in the suppression of T cell immune functions like proliferation and cytokine production. CTLA-4 is a negative regulator of anti-tumor T cells upon recognition of their ligands. Currently, therapeutic antibodies targeting CTLA-4, ipilimumab, and tremelimumab, are approved to be efficient in a wide range of hematological malignancies and solid tumors.
Colorectal Cancer
-
CRC is one of the most common malignancies in humans. It is estimated that nearly 881,000 new deaths occurred in 2018, ranking second in cancer mortality.
-
The global CRC drug market size estimate for 2018 is $994 million, and the compound annual growth rate is expected to be close to 3% between 2019 and 2023.
-
The global immunotherapy market size for CRC in 2018 is estimated at 5 billion.
Ongoing Clinical Trials
-
Currently, NO anti-GITR x CTLA-4 BsAb is being investigated in clinical trials. There is only one anti-GITR x CTLA-4 BsAb ATOR-1144, developed by Alligator Bioscience, who publicly presented their preclinical data at a recent AACR annual meeting.
-
We believe that this dual targeting strategy will provide insights into the tumor immunotherapy. In an effort to optimally leverage GITR x CTLA-4-mediated immune response, our next-IO™ GITR x CTLA-4 targeted antibody program attempts to explore the optimal combination strategy by involving other immunomodulatory agents.
Program Plan
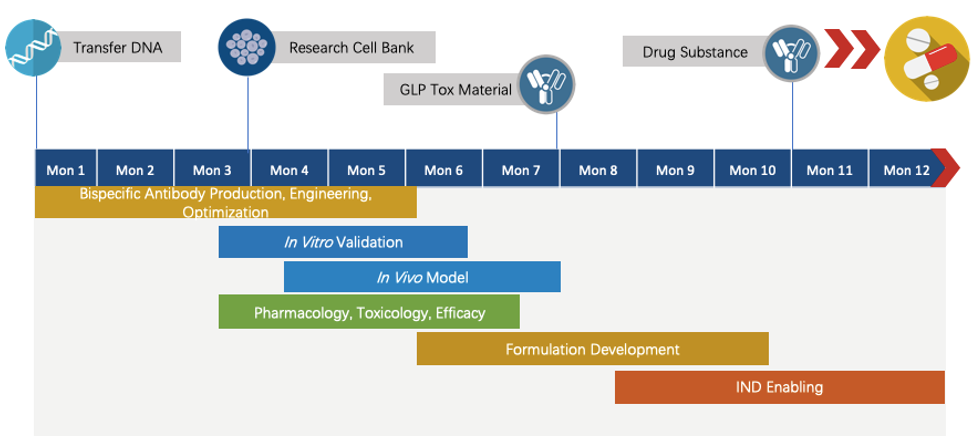
Creative Biolabs has extensive knowledge of end-to-end program development. For each program, we are committed to delivering the final complete program to our clients within 1.5 years before entering the IND stage.
Cooperation
Creative Biolabs is looking for potential partners (include but not limit to major pharma or biotech firms) to develop GITR x CTLA-4 therapeutic bispecific antibody program together. Our scientists are dedicated to bringing years of valuable experience to our partner and achieve a meaningful partnership together. For any partners interested in our Next-IO™ programs, Creative Biolabs welcomes collaboration.
Here are two ways for your choice, and please contact us for more details.
1) Collaborate with us and co-develop the programs from the discovery phase to IND enabling. Costs will be shared.
2) Become a licensed candidate for our programs.
With our quality control protocol and knowledge of global regulatory requirements, we can help our partners further their programs with more chances to succeed. Look forward to cooperating with you in the near future.
For Research Use Only | Not For Clinical Use



 Download our brochure
Download our brochure