Creative Biolabs provides in-depth molecular characterization of LPS, covering purity, structural insights, mechanistic understanding, and molecular species analysis. This enables researchers to link LPS structures to their biological activities, advancing studies in immunology and pharmaceutical development.


- Products
-
Services
- Therapeutic Antibody & Protein Development
- Synthetic Gene Circuits for Cancer Immunotherapy - Turning Cancer Cells Against Themselves
- Tumor Profiling to Guide Targeted Cancer Therapy
- Gut Microbiota-based Diagnostics Platform
- Brain-Immune-Gut Based Integrative Research
- Tumor Energy Metabolism Analysis Services
- Human Oncology Imaging Services
- Integrated Solutions for Cancer Immunotherapy Development
- Microbiological Test
- Targeting Ferroptosis Solutions in Oncology
- In Vitro ADME for Cancer Immunotherapy
- Graft-Versus-Host Disease (GVHD) Research Solutions
- Cancer Resistance Integrated Solutions
- Cardiotoxicity Analysis & Prevention Solutions
- Antibody-directed Enzyme Prodrug Therapy (ADEPT) Solutions
- Programs
- Collaborations
- Company
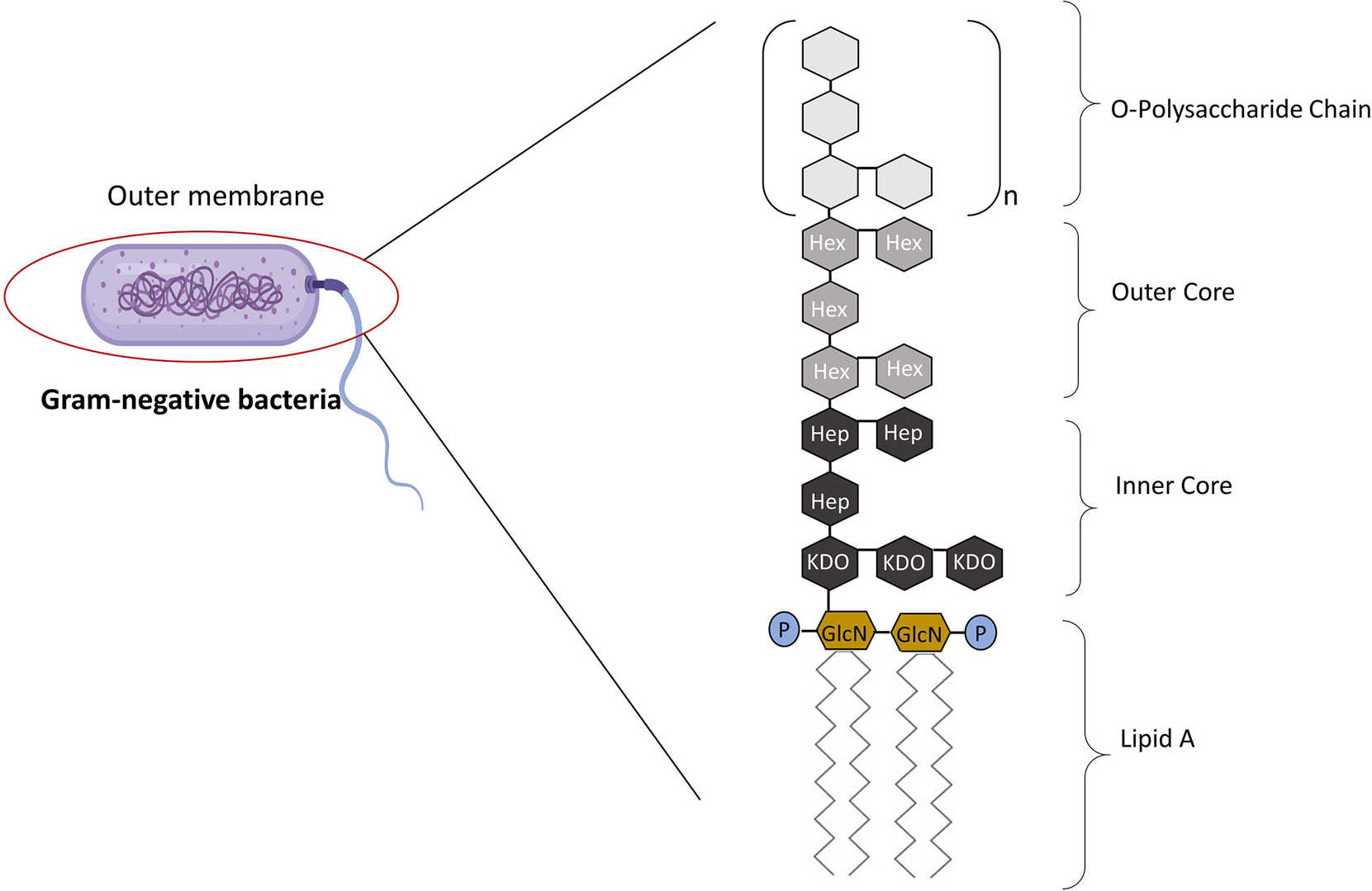 Fig.1 The Structure of LPS.1,3
Fig.1 The Structure of LPS.1,3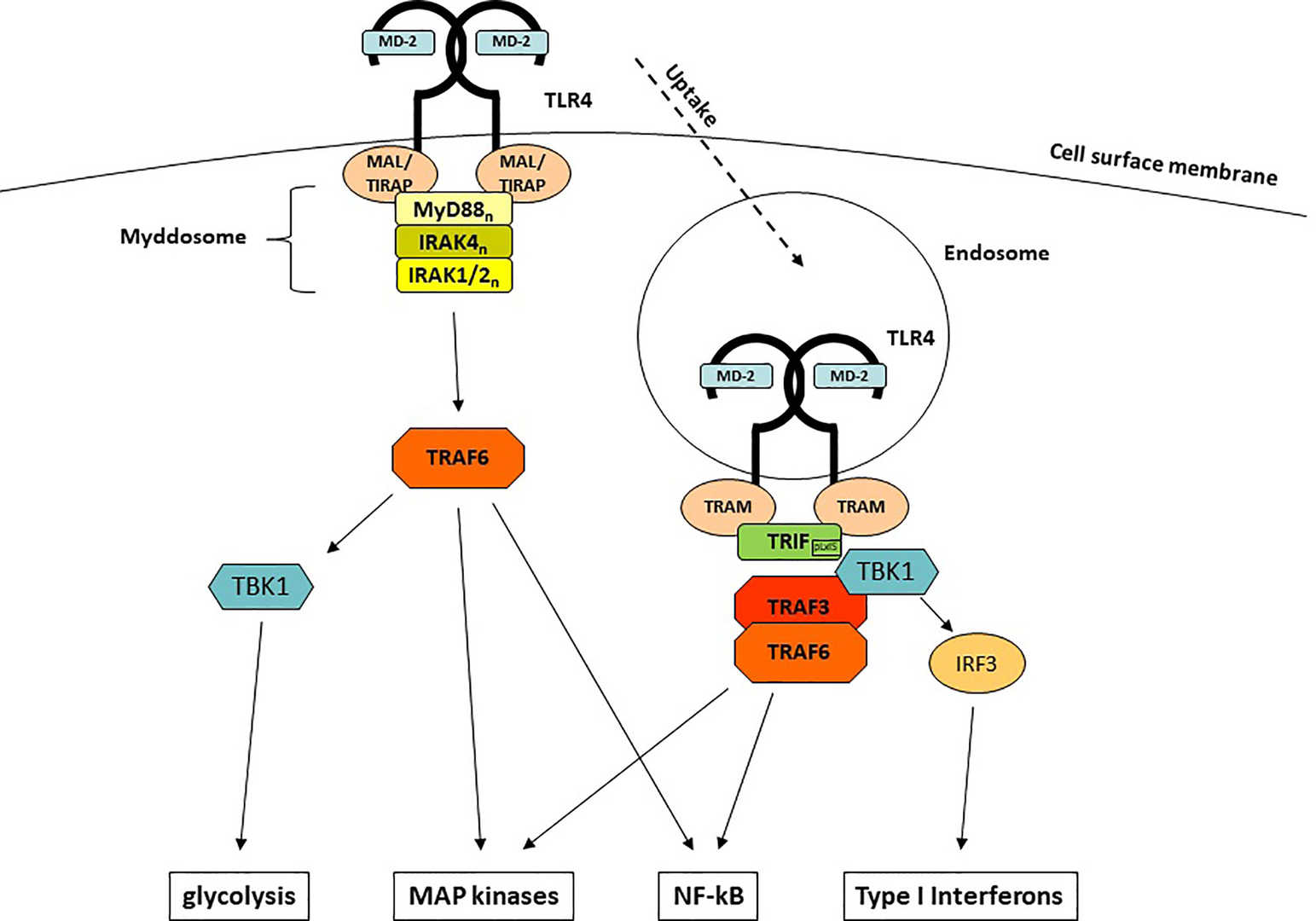 Fig.1 LPS-Induced TLR4-Mediated Signaling
Pathways. 2,3
Fig.1 LPS-Induced TLR4-Mediated Signaling
Pathways. 2,3






 Endotoxin Detection Service
Endotoxin Detection Service

 Download our brochure
Download our brochure