Endotoxin Testing
In conditions where the body is exposed to lipopolysaccharides (LPS) excessively or systemically, a systemic inflammatory reaction may occur, leading to multiple pathophysiological effects. Therefore, it is important to detect and remove endotoxin from multiple products such as injectables, drugs, and other biological and pharmaceutical products. With extensive research of endotoxin and the art technology in this field, Creative Biolabs provides endotoxin testing to ensure reliable, efficient, and sustainable testing of bacterial endotoxin.
Background
Endotoxins, also known as LPS, are derived from cell membrane of Gram-negative bacteria and are responsible for their organization and stability. Endotoxins are continuously released into the environment, which happens with cell death, growth, and division. Endotoxins do not act directly against cells or organs but through activation of the immune system, especially through the monocytes and macrophages, with the release of a range of pro-inflammatory mediators, such as tumor necrosis factor (TNF), interleukin (IL)-6, and IL-1. Therefore, endotoxins are found almost everywhere and elicit a wide variety of pathophysiological effects.
Chemical and Physical Properties of Endotoxin
Endotoxins consist of a hydrophilic polysaccharide moiety, which is covalently linked to a hydrophobic lipid moiety (lipid A). Endotoxins from most species are composed of three distinct regions: the O-antigen region, a core oligosaccharide, and lipid A. The lipid A is responsible for most of the biological activities of endotoxin which means toxicity.
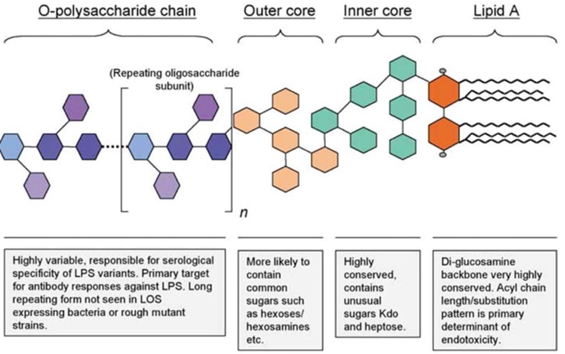 Fig.1 The general structure of Endotoxins. (Eckel, 2020)
Fig.1 The general structure of Endotoxins. (Eckel, 2020)
Properties of Endotoxin
-
Ubiquitous in nature
-
Potent toxicity
-
Stable under extreme conditions
-
Occur in the manufacturing process
Main Service
Creative Biolabs provides sensitive and accurate methods for endotoxin detection.
Creative Biolabs provides endotoxin removal services to effectively reduce the level of endotoxin in your products or other instruments.
Creative Biolabs provides LPS analysis services to assist in the study of bacterial pathogenesis.
Usage of Endotoxin Testing
-
Water for injection (WFI)
-
Injectables or parenteral products
-
Validation of depyrogenation processes
-
Radiopharmaceuticals and cytotoxic agents
Platform Advantages
-
A range of endotoxin detection and removal assays
-
Test multiple samples at the same time
-
Custom different test schemes and optimize our methods according to the characteristics of the samples
-
Provide highly sensitive detection and accurate results
-
Attach importance to the communication with the customers
Creative Biolabs has been committed to the research and development of multiple testing of bacterial endotoxin for many years. For more detailed information, please feel free to contact us or directly send us an inquiry.
References
-
Eckel, E.F.; et al. Invited review: role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J Dairy Sci. 2016, 99(8): 5967-5990.
-
Magalhães, P.O.; et al. Methods of endotoxin removal from biological preparations: a review. J Pharm Pharm Sci. 2007, 10(3): 388-404.
-
Eckel, E. F.; et al. Bacterial endotoxins and their role in periparturient diseases of dairy cows: mucosal vaccine perspectives. Dairy. 2020, 1(1): 61-90.
For Research Use Only | Not For Clinical Use


 Fig.1 The general structure of Endotoxins. (Eckel, 2020)
Fig.1 The general structure of Endotoxins. (Eckel, 2020)
 Download our brochure
Download our brochure