Cytokines serve as a type of immunoregulatory protein involved in multiple pathological states and have great potential as therapeutic drugs. Several studies showed the efficacy of cytokines in preclinical disease models. However, the characteristics of small molecular weight of cytokines, short half-life in vivo, poor pharmacokinetics, and dose-limiting toxicities, have hindered the cytokines delivery through the systemic administration routes. Therefore, a variety of molecular and formulation engineering approaches are being developed aimed at reducing therapeutic toxicity while maintaining therapeutic efficacy, such as PEG-Modified Cytokine development.
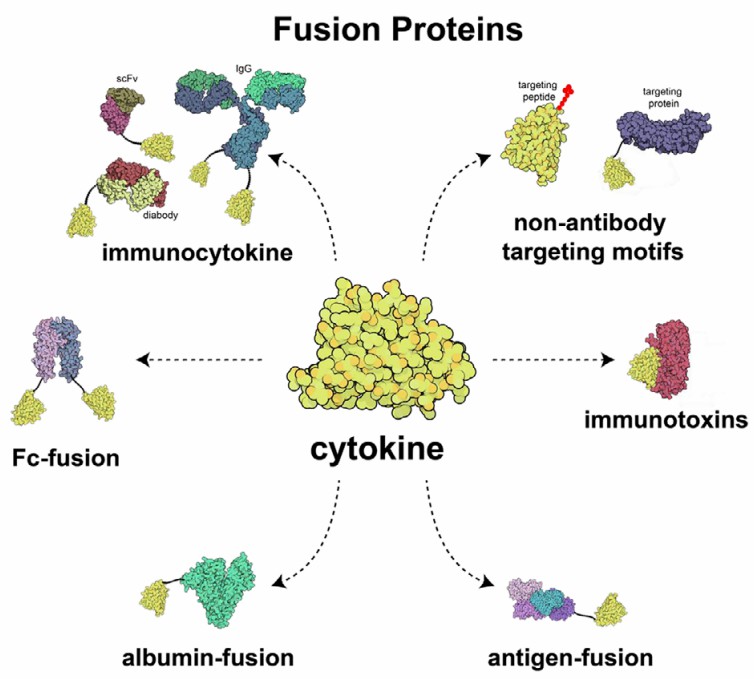 Fig.1 Schematic of several fusion proteins developed for cytokine delivery. (Pires, et al., 2021)
Fig.1 Schematic of several fusion proteins developed for cytokine delivery. (Pires, et al., 2021)
With years of experience and expertise in cancer immunology, Creative Biolabs succeed in providing comprehensive PEG-modified cytokine development services for global customers. As PEG-modified cytokine with several characteristics, such as increased plasma half-life, reduced toxicity, and improved drug stability and solubility, we perform PEG-modified cytokine development with various types of cytokines. In some cases, PEG-modified cytokine can improve therapeutic efficacy by reducing immunogenicity. In addition, our team also offers customized development services according to the special project needs of customers.
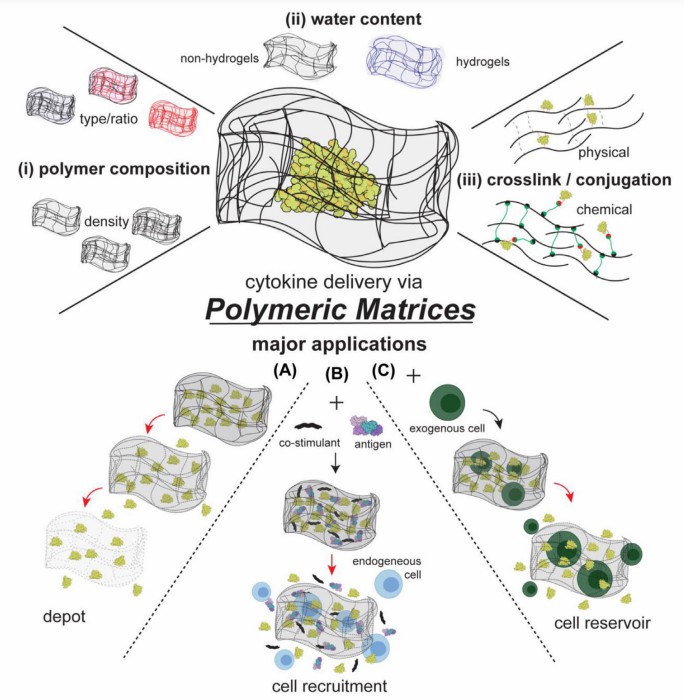 Fig.2 The main design parameters and application of polymeric matrices used in cytokine therapies. (Pires, et al., 2021)
Fig.2 The main design parameters and application of polymeric matrices used in cytokine therapies. (Pires, et al., 2021)
Here are some representative published data displays.
Representative data: In vivo animal experiments show that the cytokines encapsulated by biomaterials have a good anti-tumor effect.
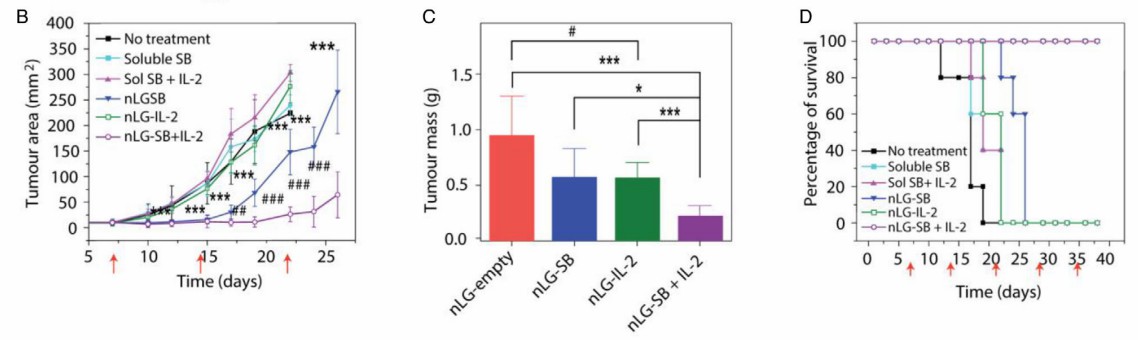 Fig.3. The effect of intratumoral administration of liposomal nanogel encapsulating a TGF-β inhibitor and IL-2 in a metastatic melanoma mouse model. (Pires, et al., 2021)
Fig.3. The effect of intratumoral administration of liposomal nanogel encapsulating a TGF-β inhibitor and IL-2 in a metastatic melanoma mouse model. (Pires, et al., 2021)
Q1: What are the regulatory considerations in the direction of clinical conversion of new PEG-modified cytokine drugs?
A1: Clinical translation design also need to take into account the pharmaceutical formulation challenges required to maintain drug storage stability and ease of administration. Optimization of biomaterial formulation can be complicated by the additional regulatory complexity for any new excipient introduction. The implementation of the new vehicle complicates the issue further as the biomaterial structure and composition of the vehicle also need to be properly maintained.
If you are interested in our Next™ PEG-Modified Cytokine Development Service, please feel free to contact us. We are looking forward to working together with global customers' attractive projects.
For Research Use Only | Not For Clinical Use