Case Study for De Novo Sequencing of an IgM Antibody
Creative Biolabs has successfully delivered results from numerous projects highlighting the ability to provide high-precision, full-coverage de novo antibody sequencing services. Here we present a case study - De Novo Sequencing of an IgM with 1% BSA, one of the most challenging projects we have successfully resolved.
De Novo Sequencing of an IgM with 1% BSA Overview
Background of the Project
Sequencing of IgM antibodies is considered one of the supreme disciplines in the field of de novo antibody sequencing. In this case study, we succeeded in solving the sequence of an IgM antibody sample that was extremely challenging.
Basic Information of the Sample
The BSA concentration is 10 mg/mL (see Figure 1) whereas the antibody concentration is only 50 µg/mL. In addition, the preparation has two light chains:
- the one that forms a functional antibody with the heavy chain;
- an aberrant light chain that is produced by many MOPC21 hybridoma.
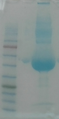 Fig. 1 10 µL of unpurified antibodies are loaded onto an SDS gel.
Fig. 1 10 µL of unpurified antibodies are loaded onto an SDS gel.
Left lane: marker. Right lane: unpurified antibody (only BSA and
BSA aggregated and degradation products were visible)
Sample Processing and Analysis
To remove the BSA, the antibody is purified using our proprietary microscale purification protocol that can recover as little as 50 μg samples in the presence of a 200-fold excess of BSA. After purification, the antibody is analyzed on an SDS-PAGE Gel.
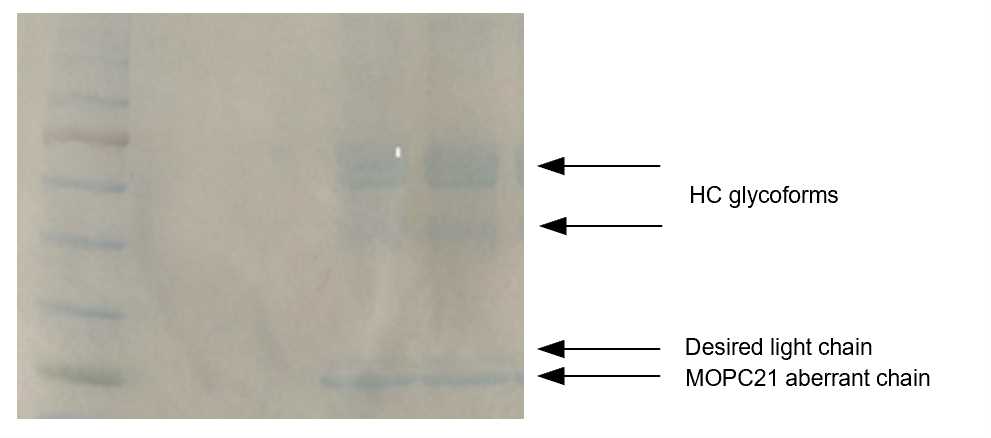 Fig. 2 SDS-PAGE analysis of the purified antibody. Left lane: Marker. Two right lanes: purified antibody.
Fig. 2 SDS-PAGE analysis of the purified antibody. Left lane: Marker. Two right lanes: purified antibody.
As shown in Figure 2, two heavy chain bands and two light chains are visible. The two heavy chain bands represent different glycoforms of the antibody. In contrast to IgG which typically has one glycan in the hinge region, this IgM has 5 glycosylation sites in the constant region. One of the two light chains is the functional one, and the other one is the aberrant chain produced by the MOPC21 hybridoma. The aberrant light chain of MOPC21 hybridoma is in 4-5 molar excess.
De Novo Sequencing and Verification
The heavy and light chain bands are excised and digested with different enzymes. After peptide recovery, the samples are characterized on an analyzer system coupled to the mass spectrometer. The high-performance high-field mass analyzer geometry and advanced signal processing technologies enable resolution of >240,000, superior spectral quality, and higher scan speed, with a mass accuracy of <3ppm.
 Fig. 3 Final sequence of the desired light chain.
Fig. 3 Final sequence of the desired light chain.
 Fig. 4 Sequence of aberrant light chain.
Fig. 4 Sequence of aberrant light chain.
 Fig. 5 Final sequence of the heavy chain.
Fig. 5 Final sequence of the heavy chain.
De novo sequencing is performed with the Database Assisted Shotgun Sequencing (DASS) algorithm and cross-validated with two other algorithms. As shown in Figures 3, 4, and 5, the full sequences of all 3 chains are reconstructed successfully.
Our Guarantee
De Novo Antibody Sequencing
- 100% coverage
- Precise discrimination of Leu/Ile
- High-throughput compatibility
- Only 50 μg samples needed
- Fast delivery
At Creative Biolabs, our expert R&D team is dedicated to helping you generate accurate amino-acid sequence information for your antibody using cutting-edge mass spectrometers and the most powerful data processing algorithms. We have sequenced over a thousand antibodies so far through this approach, and 98% of the sequenced antibodies show equal or better performance compared to the originator. Our DASS technology is proven to be a reliable tool for the discovery of novel therapeutic antibodies, as well as for the development of generic or biosimilar antibody drugs. Please do not hesitate to contact us to send your requests and we are more than happy to discuss how we could help accelerate the progress of your antibody project.