Novartis’ chimeric antigen receptor T cell therapy (CAR-T) was supported and approved unanimously (10-0) on July 12th by the U.S. FDA Advisory Committee experts. This vote marks the key milepost of this kind of experimental treatment. FDA is expected to give final approval decision on October 3rd. Carl June, a scientist from the University of Pennsylvania who led the drug development said that this may open a new chapter in CAR T immunotherapy—”a truly viable drug.”
CAR-T manufactured by Novartis is only for a small number of children and adolescents with leukemia who get no responses from standard treatment. These patients often have severe prognosis, and the key tests from nearly ten countries, 83% of the patients get into remission. After one year, 2/3 of patients are still in remission stage. Childhood leukemia is only start studied by industrial and academic field. Kite pharmaceutical company, located in Santa Monica City of California, had applied for approval in the treatment of non-Hodgkin’s lymphoma, and Novartis was following. Researchers are also exploring CAR-T for leukemia therapy and multiple myeloma. They are solving a more difficult challenge that this therapy can be used for tumor in lung or brain.
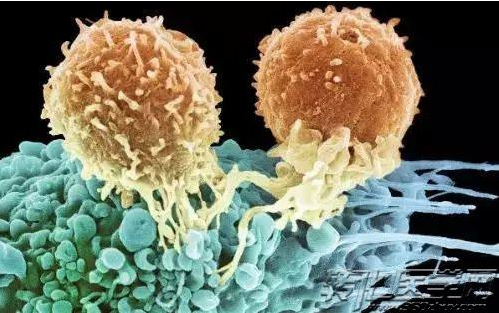
CAR-T cell therapy extracting a white blood cells (immune system guards) called T cells from patients’ blood, freezing and sending to large manufacturing plant in Morris Plains city, New Jersey. There, modified HIV fragment was used for genetic modification of T cells, so that T cell can find and attack cancer cells. The modified cell was frozen again and re-injected into the patient. Once in the body, the number of T cells increased.
Doctors and researchers were excited obviously. Oncologists at University of Pennsylvania and Stephen Schuster who led Novartis Lymphoma Research said, “we are saving those patients who let all the scientists were at a loss to maintain their lives three or four years ago.” Novartis’ lymphoma research and Kite’s pharmaceutical test showed that drug treatment can make about 1/3 of patients with advanced disease (all treatment options have been exhausted) eased.
However, with enthusiasm, the safety, cost and complexity of therapy are urgent.
The company has not disclosed the price of treatment, but analysts predict that the disposable infusion is about $300 thousand to $600 thousand. Brad Loncar (a company whose investment fund is focused on the development of immunotherapy) hoped that the spend will not lead to a rebound. He said, “CAR-T is not EpiPen. It is really beyond the limit and the frontiers of science”.
Safety concerns
However, the biggest concern is security issue. Accelerated immune system becomes an effective anti-cancer agent, but it was also a danger on patients. Many serious side effects need us to worry about. Stephan Grupp, from Children’s Hospital of Philadelphia, said, “safely treatment is the core; validity problems will be resolved, but safety requires a lot of attention.” Grupp was the directory leading pediatric research and early test of Novartis hospital cancer immunotherapy program.
One of the most common side effects are cytokine release syndrome, which can cause high fever and symptoms of anxiety. In some cases, it may be too dangerous to patients in intensive care. Another major concern is neurotoxicity, which may cause temporary confusion or potentially fatal brain edema. Juno Therapeutics shut down its CAR-T program after the death of five patients with brain edema. Novartis official said there was no brain edema in the test of Novartis.
In order to ensure the safety of patients, Novartis had no typical plans to promote products as widely as possible. Instead, the company will appoint 30 to 35 medical centers to implement treatment. Many medical staff participated in clinical trials, and got a lot of training of Grupp.
With the terrible experience from Emily Whitehead 5 years ago, Grupp said he and his staff understood the side effects of CAR-T therapy and how to handle them. Emily had recurred two times of acute lymphoblastic leukemia with routine treatment. Grupp suggested to her parents to let Emily be the first one receiving the experimental treatment. After treatment, the Emily’s temperature rose, blood pressure was dropped, and stayed in ICU of hospital ultimately for two weeks. When Grupp assured that Emily was not likely to live another day, he got the laboratory test results which showed that the surge in interleukin 6 protein made her immune system attack the body. The doctor decided to give a tocilizumab immune suppressing drugs. She improved in a few hours. On the second day, she woke up on her seven birthday. The tester showed that her cancer had disappeared.
The history of CAR-T
CAR-T cell therapy’ authorization will represent the second major advance in the treatment of immune less than ten years. In 2011, FDA approved the first preparation of new drugs called immune check point. From then on, five other drugs were also approved. There is a big difference between the two treatments. Checkpoint inhibitors are for solid tumors, such as malignant melanoma, lung cancer and bladder cancer, while CAR-T cell therapeutics is for blood disease. Although checkpoint inhibitors are readily available, each patient receives the same drug, while CAR-T cell therapies is based on a custom body. Many immune treatment experts believe that when researchers find the combination method, cancer will achieve the biggest progress.
For University of Pennsylvania team, CAR-T cell story can be traced back to a few decades ago. There was known as a National Naval Medical Center, where June and Bruce Levine researcher engaged in the study of new HIV treatment. In this process, they came up with an enhanced T cell to make it more powerful and more adequate. Two partners moved to Philadelphia in 1999 for cancer research. Two years later, June’s wife died of ovarian cancer, which motivated him to work harder in this field. In the next few years, researchers around the country obtained a T cell and a series of attractive discoveries.
About 2010, Bill Ludwig became the first patient receiving CAR-T cell therapy in the University of Pennsylvania. Another two people received treatment in the near future. One is still in remission, and the other one died of recurrence. After the three patients, researchers at University of Pennsylvania spent all money but cannot have more treatment. In order to enhance people’s interest for this therapy and access to funds, they decided to publish research results. In August, 2011, an article published on new England Journal of Medicine (NEJM) caused a storm to bring new resources to them. The pediatric trial and success of Emily therapy opened a new spring. After six months, the University of Pennsylvania transferred the technology to Novartis in exchange for financial support, including a new cell manufacturing facility.
FDA’s approval seems imminent, and researchers have played an important role in the development and testing of treatments.
Reference material:
First gene therapy – ‘a true living drug’ – on the cusp of FDA approval – The Washington Post