All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
As a leading technology provider, Creative Biolabs has established CellRapeutics™ Chimeric Antigen Receptor (CAR) Technology platform. Our elite scientists have broad experience in CAR construction, which can supply the best service for you.
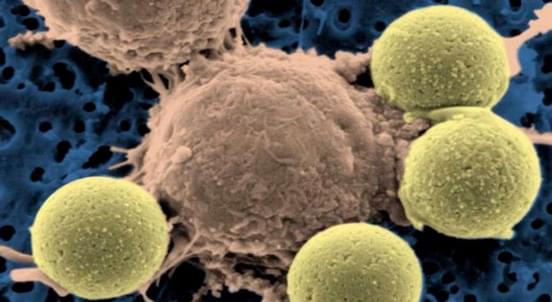
scFv Production in CAR Technology
The typical structure of a CAR molecule includes a single chain variable fragment (scFv), a spacer, a transmembrane domain (TM) and an intracellular signaling domain. The scFv is derived from monoclonal antibody (mAb), which can specifically recognize the target protein on tumor surface and subsequently transduct activation signal into CAR-T cell. With our one-stop solution, we can carry out scFv generation from hybridoma cell line (full length of monoclonal antibody) through the converting full immune globulin (monoclonal antibodies) into a scFv using a short flexible linker or phage display library which has nearly 1.0 × 108 individual candidate clones. Furthermore, synthesis a scFv gene based on the existed sequence from customer is also an available service.
Creative Biolabs has established a state-of-the-art CAR-T library technology based on T cell display. CAR genes with diverse antibody fragments (e.g. scFv, Fab, VHH, scFab) against a certain target are constructed into vectors to form the CAR-T libraries with capacity around 108. T lymphocytes will display these antibodies on the surface and high-throughput screening approaches can be performed. Due to the linkage between the T cell activation and the expression and function of the CARs, we would obtain functional CARs with high affinities ranging from 10 pmol to nmol. Notably, we have generated several CAR-T libraries not only for blood cancer antigen CD19, but also for solid tumors against different antigens, such as Her2, Her3, EGFR, FGFR1, VEGFR, etc. The selected stable clones can be used in clinic trials immediately, thus making this technology more powerful and attractive in CAR-T cell immunotherapy.
There're four generations of CAR products available in Creative-biolabs. The first generation CAR consisting of basic elements has been widely applied in early cancer immunotherapy clinical trials. Different from the first generation which has one intracellular signaling domain like CD3ζ or or FcεRIγ, our second-generation CAR is composed of an activating domain plus a co-stimulatory signal domain such as CD28 or 4-1BB, the structure of which can support longer antitumor effect. Similar to this strategy, two different co-stimulatory signaling regions are constructed into the third generation CARs in order to promote the T cell activation signal and enhance the proliferation and survival of CAR-T cells significantly. Furthermore, the fourth generation CAR construct is engineered with an inducible expression unit such as a cytokine (like IL-12), which can effectively overcome the "on-target, off-tumor" drawbacks via this inducible transgenic cytokine gene.
With our experience in CAR technology field, we can provide innovative, high quality CAR application platform such as animal models (e.g. murine, canine and non-human primate (NHP)). Up to now, we have successfully developed CAR-T cells or -NK cells and evaluated their anti-tumor effect and potential safety profile in these animal cancer models, which can greatly support your preclinical trial researches. Meanwhile, these animal toxicity testing system can also identify and assess the safety profile of CAR modified T cells or NK cells, which including "on-target, off-tumor" toxicities targeting normal tissues and cytokine-release syndromes.
Our bispecific CAR-T construction service includes cocktail CAR, trans-dual specificity CAR and TanCAR. The cocktail CAR consists of two tandem scFvs connected by a linker, which can recognize two different tumor antigens so as to enhance the specificity and facilitate the activation of T cells, especially when one cancer antigen is downregulated or mutated. Alternatively, we can also coexpress two distinct CAR constructs simultaneously within T cells, such as trans-dual specificity CAR and TanCAR. With the help of these advanced approaches, we can greatly increase the specificity of CAR to tumor cells whereas decrease its toxicity to normal tissue.
GMP Manufacturing and Clinical Trials
To facilitate the CAR production for late-stage cancer patients in clinical test, Creative Biolabs has developed and optimized our good manufacturing practices (GMP) compliant CAR construction procedures. We assure that these genetically engineered T cells can meet all quality control standards and the FDA clinical trial criteria. In collaboration with many academic and industrial groups, Creative Biolabs can offer the best service for every customer.
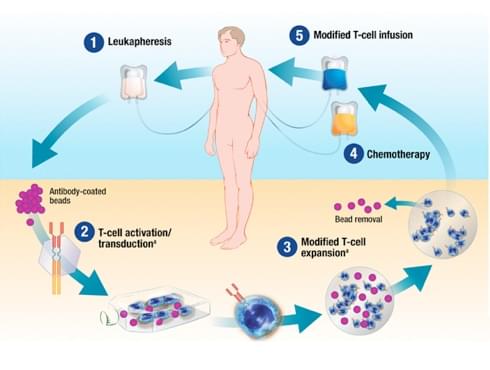
BL Levine. Performance-enhancing drugs: design and production of redirected chimeric antigen receptor (CAR) T cells. Cancer Gene Therapy. (2015) 22, 79–84.
Based on our advanced CellRapeutics™ chimeric antigen receptor (CAR) technology platform, Creative Biolabs is dedicated to providing CAR-T cell glycosylation service and Luminex assay service for monitoring CAR-T cytokine profiles. For more detailed information, please feel free to contact us or directly sent us an inquiry.
For more interests of CAR-T therapy, please follow up with our updating CAR T Technology Publications.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION