Cluster of differentiation 47 (CD47) is a widely expressed glycoprotein on the cell surface, which can interact with a variety of intracellular and extracellular proteins such as thrombospondin-1 (TSP-1) and signal regulatory protein α (SIRPα) and participate in the regulation of various signaling pathways that play an important role in the occurrence and development of many diseases such as tumors, cardiovascular diseases, and autoimmune diseases. The understanding of the CD47 structure, expression, interaction with other proteins, related signaling pathways, and the relationship with diseases, can help the development of therapeutic strategies targeting CD47.
Previous studies showe that CD47 interacts with integrins αvβ3, αⅡ, bβ3, and α2β1 to regulate integrin function and cell response to extracellular matrix proteins containing arginine-glycine-aspartic acid (RGD). Therefore, it is called integrin-related protein. CD47 is highly expressed in a variety of tumors, such as ovarian cancer, leukemia, breast cancer, colon cancer, bladder cancer, glioblastoma, hepatocellular carcinoma, and prostate tumor. Studies also indicate that anti-CD47 antibodies can stimulate macrophages to clear tumor cells, so CD47 has become a research hotspot in recent years.
1 Structure, Expression, and Interaction of CD47
1.1 Structure
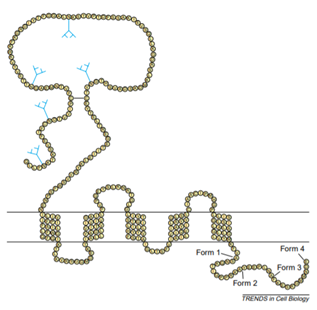
CD47 belongs to the immunoglobulin superfamily, which has two isomers, one containing N glycosylation and the other containing glycosaminoglycan modifications at its 64 and 79 serines. The CD47 structure consists of three domains, the N-terminal extracellular immunoglobulin variable (IgV) domain, the highly hydrophobic five-transmembrane domain, and the short variable C-terminal intracellular domain, as shown in figure 1.
Fig. 1. Schematic diagram of CD47 structure. (TRENDS in Cell Biology)
CD47 interacts with its ligands TSP1 and SIRPα through the extracellular IgV domain. The IgV domain is modified by N-linked glycosylation and O-linked glycosylation, which is necessary for the interaction between CD47 and TSP. There is a long disulfide bond between the amino acid residue Cys33 on the IgV domain and the amino acid residue Cys263 on the last extracellular loop of the transmembrane domain, which is necessary for the binding of CD47 ligands, signal transduction, and localization to the lipid raft. There are four types of alternative splicing of the intracellular domain. According to the difference in gene structure and peptide sequence, it can be divided into type Ⅰ, type Ⅱ, type Ⅲ, and type Ⅳ. The number of amino acids varies from 3 to 36. At present, type Ⅱ is the most important and most expressed splice form, followed by type Ⅳ, which is mainly expressed in the brain and peripheral nervous system, while type I is only significantly expressed in keratinocytes, and type Ⅲ is mainly related to memory consolidation.
1.2 Expression
CD47 is expressed on the surface of almost all cells, including red blood cells and platelets, and its expression level varies according to the immune or pathological status of the body. CD47 is highly expressed various tumors, and the phagocytosis of tumors can be enhanced by blocking CD47. Xu et al. found that anti-CD47 therapy can increase the phagocytic function of macrophages, reduce tumor weight, and inhibit spontaneous metastasis in osteosarcoma xenotransplantation model. Zhang et al. found that blocking CD47 can enhance the phagocytosis of macrophages to tumor cells, reduce tumor load, and improve the survival rate of patients with glioblastoma. Similarly, Yoshida et al. found that blocking CD47 can enhance the phagocytosis of macrophages in gastric cancer cells.
All these suggest that CD47 may be an important survival signal molecule of tumor cells and is closely related to the occurrence and development of tumor. In the process of immune response, the expression of CD47 on the surface of CD4 effector T cells is increased, which prevents memory T cells from being cleared by macrophages and prolonging their life cycle. Similarly, the high expression of CD47 on the surface of hematopoietic stem cells (HSCs) also protected it from macrophage phagocytosis. When the expression of CD47 on the surface of CD34+ CD38- HSCs is selectively down-regulated, hemophagocytic lymphohistiocytosis (HLH) may occur.
1.3 Interaction
CD47 mainly interacts with three extracellular ligands through the extracellular IgV domain, which are CD47-TSP1, CD47-SIRP α, and CD47-CD47, respectively. Due to the small intracellular domain of CD47, only a limited number of intracellular signaling proteins interact with CD47, mainly BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3) and protein-linking integrin-associated protein and cytoskeleton (PLIC). The transverse interaction between CD47 and other transmembrane proteins may play an important role in the signal transduction pathway of CD47, such as integrin αvβ3, vascular endothelial growth factor receptor 2 (VEGFR2), Rh blood group antigen complex, CD47, factor associated suicide (Fas), and CD14.
1.3.1 Extracellular Interaction
The first found endogenous ligand of CD47 was TSP1, a member of the TSP family, including TSP1~TSP5. TSP1 is a transient expression cell matrix protein, which in different environments, regulates cell function by combining with cell surface receptors or other extracellular matrix components. TSP1 has a large structure and contains multiple domains, which enable it to interact with various cell surface receptors and extracellular matrix, including integrins (mainly β1 and β3), CD47, CD36, heparan sulfate proteoglycan (HSPG), low density lipoprotein receptor-related protein 1 (LRP1), and very low-density lipoprotein (VLDL) receptor. TSP1 binds to CD47 through the VVM sequence on its C-terminal binding domain, and then affects a variety of cell functions, such as cell migration and adhesion, cell proliferation and apoptosis, and regulation of angiogenesis and inflammation.
The second found endogenous ligand was SIRPα. SIRP α, which belongs to the immunoglobulin superfamily, is a transmembrane protein that can be expressed at different levels on the surface of myeloid cells. The expression of SIRPα on the surface of phagocytes seems to be very stable and will not be affected by the degree of inflammation, while the expression of CD47 changes with different immune status or diseases. The extracellular N-terminal of SIRPα contains three Ig-like domains and its cytoplasmic C-terminal contains two immunoreceptor tyrosine-based inhibitory motif (ITIM). ITIM mediates the recruitment and activation of SHP-1 and SHP-2, which affects the intracellular signal transduction pathway. SIRPα binds to the IgV domain of CD47 through its IgV domain of N-terminal, and the interaction between them plays a role in controlling cell phagocytosis and can produce a signal of “don’t eat me”.
Adhesion between cells requires the presence of CD47 but not its ligand, which indicates that the IgV domains of CD47 on the two cells also has homotypic binding. However, there is insufficient evidence that the interaction between CD47s can produce signal transduction.
1.3.2 Membrane Surface Interaction
CD47 interacts with integrin αvβ3 and other integrin subtypes, such as αⅡbβ3, α2β1, αLβ2, and α4β1. CD47 triggers subsequent intracellular signal transduction by activating integrin, which is independent of its transmembrane domain. CD47 binds to these integrins and can change the signal targets of integrins, such as focal adhesion kinase and paxillin. The CD47-integrin complex is also associated with trimeric G-protein and participates in the regulation of cAMP signal transduction.
CD47 can interact with VEGF and VEGFR2, while TSP1 can suppress the downstream signal cascade of VEGF through CD47. In addition, co-immunoprecipitation and fluorescence resonance energy transfer showed that CD47 could interact with VEGFR2 directly, but this interaction would be destroyed by the binding of TSP1 and CD47 and the binding of VEGF to VEGFR2, thus inhibiting the signal transduction of VEGFR2.
1.3.3 Intracellular Interaction
Using CD47 transmembrane domain and C-terminal tail as bait for yeast two-hybrid system, BNIP3 was found to be a cytoplasmic binding ligand from human lymphocyte cDNA library. Reverse inhibition of BNIP3 can inhibit CD47-mediated apoptosis. Activation of CD47 with TSP1 can induce BNIP3 transfer to mitochondria, resulting in cell death. However, the combination of SIRPα and CD47 could not cause BNIP3 metastasis.
Using the integrin-related protein-2 (IAP2) and IAP4 splicing isoforms of the CD47 cytoplasmic tail as bait for the yeast two-hybrid system, two other ubiquitin-related proteins were identified as CD47 cytoplasmic binding ligands, which are integrin-related proteins and cytoskeleton proteins PLIC1 and PLIC2, respectively. Follow-up studies found that PLIC1 binds to G-protein βγ subunit (Gβγ), which makes CD47 bind to trimeric G protein and regulate its downstream signal pathway.