Recently, AstraZeneca reached a global development and commercialization agreement of up to US $6 billion with the Daiichi Sankyo Company Limited for the development and commercialization of the antibody-drug conjugate (ADC). This makes ADC drugs become the focus of attention again.
ADC conjugates bioactive chemical small molecular drugs to monoclonal antibodies that can accurately target antigens through linkers, so as to solve the mistaken killing effect of chemotherapeutic drugs, improve the therapeutic effect, and greatly improve the drug specificity to cancer cells. Thus, it is called the “medicine missile”.
Although ADC drugs have all the advantages of large molecules and small molecules, they still face great challenges in drug design and manufacture because of their complex structure. However, in recent years, the novel technology of targets, small molecular toxins, antibodies and linkers has accelerated the listing of ADC drugs. Let’s talk about this “medical missile”.
A Survey of ADC History
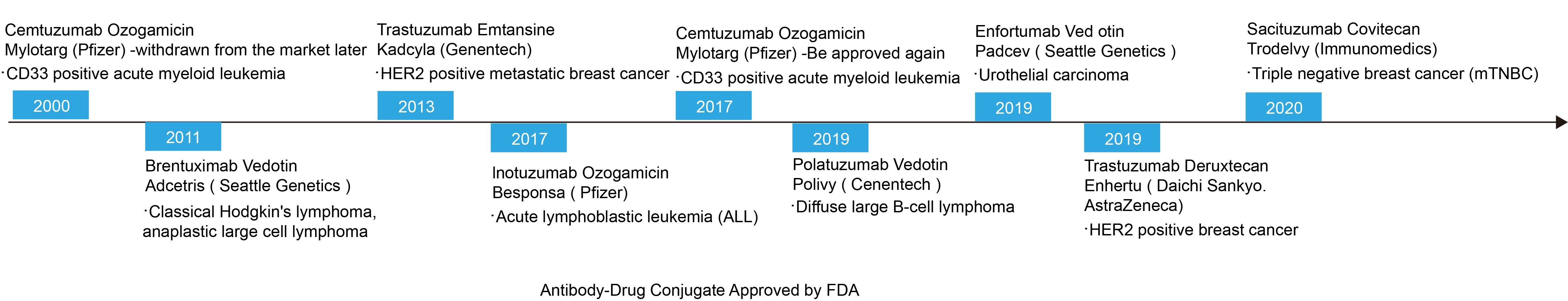
The concept of ADC drug has a history of one hundred years, and related drug experiments were recorded in the 1960s. Because of the complexity of its pharmacological properties, the first drug did not enter the market until the 21st century.
In 2000, Pfizer’s Mylotarg, the world’s first ADC drug, was approved to be listed on the market. Unfortunately, the drug failed to show its efficacy later, but raised some concerns about its safety, so Pfizer took the initiative to withdraw it from the market. After 2000, ADC drugs entered a “silent period” for a decade, and it was not until a decade later that there was a second product on the market.
Since then, with the deepening of research and the updates of technological, the ADC development has gradually received renewed attention, and last year ushered in a round of “outbreak”. Three drugs have been approved to go on the market, causing widespread concern in the industry. According to statistics, up to the first quarter of 2020, there are 311 active ADC drugs in the world, 33 clinical phase II and phase III R & D pipelines, and product research and development are concentrated in the field of oncology and immunity.
At present, the main research and development of ADC drugs comes from the United States,and the candidates number are 139, accounting for more than half of the global candidates.
Four key points for ADC development
Targets, antibodies, cytotoxins and linkers are four important aspects that determine the success of ADC drugs. In the past two decades, through continuous research in these four aspects, the development technology of ADC drugs has been updated to the third generation.
The target selection of ADC is similar to that of monoclonal antibody drugs, that is, the antigen needs to be highly expressed in cancer cells and low in normal cells, thus reducing miss toxicity. With the continuous discovery of targets in recent years, ADC drugs also have more target choices. At present, the main targets are EGFR, Her2, CD30, etc. In addition, CD138, CD37, mesothelin are also considered as targets.
Antibodies play the role of “precise guidance” in ADC drugs. High targeting and minimum immunogenicity are the main characteristics of antibodies in ADC drugs. At present, all ADC in clinical trials use human IgG molecules, which has high affinity and long circulatory half-life in the blood, and most choose IgG1 subtype.
Linker is the key factor determining success of ADC drugs and also is the biggest challenge facing enterprises. Linker selection and design is most important to be able to release effector molecules (cytotoxic) effectively after reaching target within vivo. Currently, the linker can be divided into pyrolysis type and non-cracking type. Creative biolabs’s proprietary DrugLnk organic synthesis platform can provide ADC developers unique customized design and synthesis services to accelerate drug listing process.
Cytotoxin is ADC’s “ammunition”, which is key to affect ADC activity. High cytotoxicity low immunogenicity and stable circulation in systemic circulation are the characteristics that they need to have. The cytotoxic drugs used in early development include alkaloids, methotrexate, anthracycline antibiotics, and toxoids. These cytotoxic drugs limit their R&D due to their lack of effective load lack of targeted specific toxicity and heterogeneous distribution of cytotoxic cells. Commonly used cytotoxins in currently drug development include anti-DNA classes (calicheamicin), anti-tubulin (maytansinoids and auristatins).
Opportunities and Challenges
By the end of July this year, eight new ADC drugs had been approved and put on the market around the world. In April 2020, FDA in the United States recently approved the listing of Immunomedics’s TROP-2 ADC, and GSK’s ADC is expected to be approved in the near future. These new ADC drugs on the market, aimed at a wide range of cancer targets, have achieved very good results in the treatment of a variety of indications, including hematoma, solid tumor and so on.
In addition to the outbreak of indications, the combination of ADC and immunotherapy is also favored by drug companies. 40 clinical trials have been opened. The most commonly used immunotherapy is PD-1 antibody, followed by CTLA-4 and PD-L1 antibody. A number of combination therapy trials have entered the clinical stage.
In addition to cancer, ADC drugs are also actively exploring indications other than oncology, including immunology, anti-infection, endocrine / metabolism and other diseases. In the treatment of these non-cancer areas, the drug load coupled to ADC is no longer limited to cytotoxic drugs. According to incomplete statistics, in the treatment of non-cancer indications, the vast majority of the load is non-cytotoxic drugs, accounting for 90% of research and development projects. These non-cytotoxic drug loads include immunomodulators, enzymes, and innovative treatment models such as antisense oligodeoxynucleotides and siRNA.