On Sept. 12, the Wall Street Journal reported that Gilead was reaching an acquisition agreement of more than $20 billion with Immunomedics to expand its oncology product pipeline, including trodelvy (sacituzumab govitecan), an antibody-drug conjugate (ADC) approved by FDA for the treatment of triple-negative breast cancer.
Trodelvy is the first ADC approved by FDA for the treatment of triple-negative breast cancer and the first approved ADC targeting human trophoblast surface antigen 2 (Trop-2). It is mainly used to treat adult patients with metastatic triple-negative breast cancer who have received at least two previous treatments. In a multicenter, single-arm, II phase clinical trial, the ORR of 108 (median, range: 2-10) patients with triple-negative breast cancer who had previously received three-line treatment was 33.3% (95% CI: 24.6%~43.1%), and the median DOR was 7.7 months (95% CI: 4.9~10.8 months).
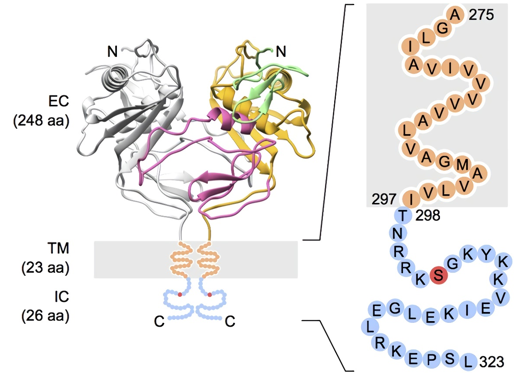
Fig.1 Domain organization of TROP2. (Pavšič, 2015)
Trop-2 is highly expressed on the surface of breast cancer, cervical cancer, colorectal cancer, kidney cancer, liver cancer, lung cancer, pancreatic cancer, prostate cancer and other tumor cells, especially in patients with triple negative breast cancer, the expression rate of Trop-2 is as high as 90%, but the expression of Trop-2 in normal tissues is limited. Therefore, Trodelvy can target govitecan, the active metabolite of irinotecan, which is commonly used in clinical chemotherapy, to solid tumor lesions by specifically targeting monoclonal antibody sacituzumab, exerting the effect of chemical toxicity.
Triple-negative breast cancer accounts for 12% of all breast cancers. Compared with other types of breast cancer, triple-negative breast cancer is more common in young women, prone to recurrence and metastasis, and high mortality. Triple-negative breast cancer is insensitive to hormone therapy and targeted therapy (such as Herceptin), and the only treatment available is chemotherapy, but most patients quickly develop drug resistance and have a poor prognosis. If it spreads to other parts of the body, the survival time is usually only 12-15 months. Therefore, there is an urgent need to develop new effective treatment strategies. In addition to triple negative breast cancer, trodelvy is also developing indications for urothelial cancer, lung cancer, endometrial cancer and liver cancer.
ADC drugs are also popular for new drug approvals and mergers and acquisitions in the past two years. Following the $6.9 billion deal on DS-8201 (HER2 ADC) between AstraZeneca and Daiichi Sankyo Company Limited in March 2019, AstraZeneca again reached a global collaborative development agreement for DS-1062 with a total transaction of US $6 billion, including a down payment of US $1 billion, on July 27th this year.