Neovascular age-related macular degeneration (nAMD ) and diabetic macular edema (DME) are retinal diseases that are the two leading causes of vision loss, affecting more than 40 million people worldwide. Roche recently announced that the European Commission (EC) has approved the bispecific antibody drug, Vabysmo (Faricimab), for the treatment of visual impairment due to nAMD (or wet AMD) and DME. Previously, this drug was approved by the US Food and Drug Administration (FDA) in January of this year.
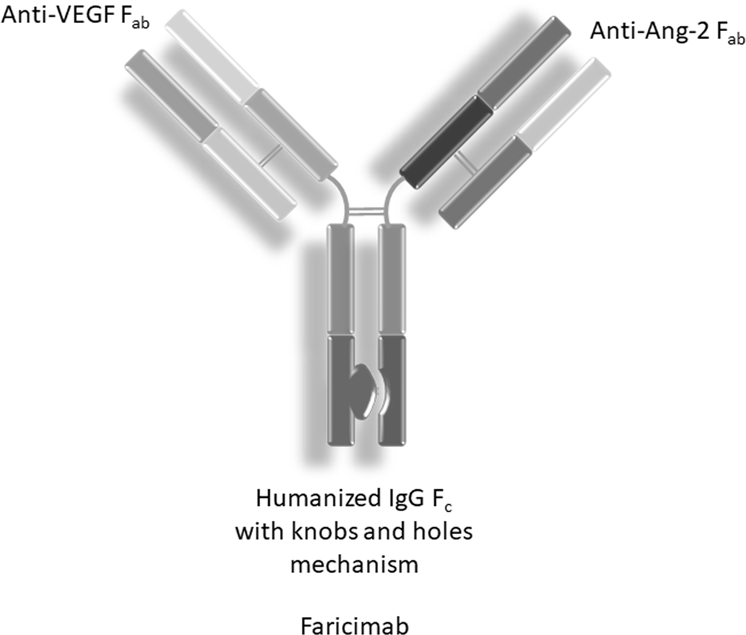
Fig. 1 Faricimab structure. (Sharma, 2020)
Standard care for nAMD and DME typically requires eye injections every 1-2 months. While anti-vascular endothelial growth factor (VEGF) monotherapy injections can significantly reduce vision loss in patients with nAMD and DME, the treatment burden associated with frequent eye injections and physician visits can lead to under-treatment and may result in less-than-optimal vision outcomes.
Vabysmo is the first bispecific antibody approved for ocular therapy and marks a significant advance for nAMD and DME with reduced injection frequency compared with the current standard of care. Vabysmo is the only injectable ophthalmic drug with Phase III clinical studies supporting treatment intervals of up to 4 months for patients with nAMD and DME. Over time, it may be able to improve and maintain visual acuity and anatomy with fewer ocular injections. As a result, Vabysmo may provide a more relaxed treatment plan for patients, caregivers, and healthcare systems.
Vabysmo is also the first drug designed to target the 2 unique signaling pathways that drive retinal disease, and its active pharmaceutical ingredient, faricimab, is the first bispecific antibody designed specifically for the eye. Unlike current treatments for DME and nAMD that inhibit the VEGF pathway, faricimab targets 2 distinct pathways, via angiopoietin-2 (Ang-2) and vascular endothelial growth factor A (VEGF-A), that drive a variety of retinal diseases, including nAMD and DME.
Ang-2 and VEGF-A contribute to vision loss by destabilizing blood vessels, causing new leaky vessels to form, and increasing the inflammatory response. By blocking these 2 pathways simultaneously, faricimab aims to stabilize blood vessels and reduce inflammation and leakage more than inhibiting either pathway alone. This may improve visual acuity more than anti-VEGF therapy alone, and thus reduce the frequency of eye injections required.
The EU approval, based on the results of four Phase III clinical studies conducted for 2 therapeutic indications (nAMD and DME) involving 3220 patients, one-year results from YOSEMITE and RHINE trials for nAMD treatment, and 2-year results from YOSEMITE and RHINE trials for the DME treatment. The results of the 4 studies consistently showed that, compared with Eylea (aflibercept, abciximab), whose dosing regimen is of every 2 months, Vabysmo’s dosing regimen can be given up to every 4 months, achieving similar visual acuity gains and anatomical improvements.
Overall, 2-year data from all four studies showed that more than 60% of patients treated with Vabysmo were able to extend the treatment interval to every 4 months while improving and maintaining their vision. In addition, over a period of up to 2 years, patients with nAMD and DME treated with Vabysmo had a median of 33% (10 vs 15 injections) and 21% (11 vs 14 injections) fewer injections than Eylea, respectively.
Reference
1. Sharma, Ashish, et al. “Faricimab: expanding horizon beyond VEGF.” Eye 34.5 (2020): 802-804.
