Immunotherapy based on immune checkpoint blockers has shown great therapeutic potential by blocking the interaction between checkpoint receptors on immune cells and their ligands on tumor cells, thereby restoring T cell function to eradicate tumors. Despite the encouraging antitumor effects of anti-programmed cell death protein-1 (PD-1) antibodies (aPD-1), their clinical application is limited by inadequate immune response rates and various immune-related adverse events.
Systemic administration of antibodies results in limited accumulation in tumor areas and may cause off-target effects and toxic side effects by binding to normal tissues. Studies have confirmed that the therapeutic efficacy of immune checkpoint inhibitors largely depends on their delivery modality. Appropriate targeted delivery systems can improve local drug concentrations in the tumors, enhance the antitumor immune effect, and reduce the required dose to minimize toxicity to normal tissues.
Recently, Professor Quanyin Hu’s team at the University of Wisconsin-Madison published a paper titled “Active recruitment of anti-PD-1-conjugated platelets through tumor-selective thrombosis for enhanced anticancer immunotherapy” in Science Advance.
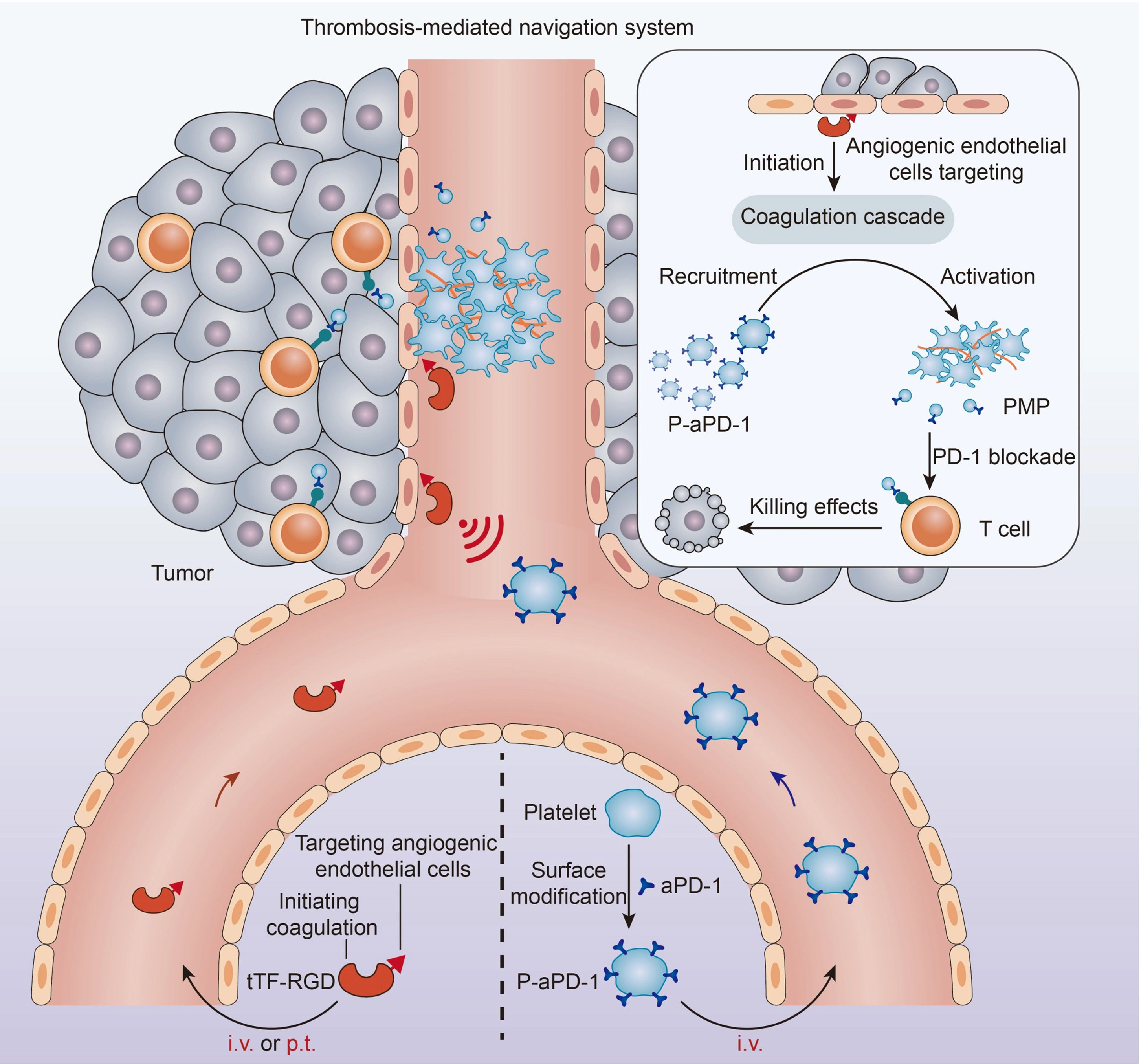
Fig. 1 Schematic of the thrombosis-mediated navigation system for P-aPD-1 (Wang, 2023)
Cells are naturally recognize specific physiological signals, for example, platelets can actively aggregate at sites of vascular injury to participate in hemostasis, while also responding to coagulation signals and participating in thrombosis. Taking inspiration from this, the team created an artificial “cellular hive” at the tumor site that attracts aPD-1 antibody-modified engineered platelets (P-aPD-1) to the tumor site, which can locally release immunotherapeutic agents to improve the targeting and controllability of cell-based delivery system.
To achieve this goal, the research team mimicked tissue factor (TF) to synthesize a fusion protein consisting of tissue factor fragment-RGD peptide (tTF-RGD) to initiate tumor-specific coagulation. As one of the initiators of coagulation, TF activates the exogenous coagulation pathway and recruits a large number of platelets to form thrombi. In this design, tTF-RGD targets tumor neovascularization with the affinity of RGD to integrin αvβ3 overexpressed on tumor neovascular endothelial cells, mimicking TF function and locally triggering the coagulation cascade signal. The subsequently injected P-aPD-1 can enrich the tumor site in response to this physiological coagulation signal. Tumor-specific coagulation further triggers platelet activation and secretion of platelet-derived vesicles (PMP) to release aPD-1 antibodies, thereby restoring the killing effect of T cells on tumor cells.
The experimental results showed that both peritumoral injection and intravenous injection of tTF-RGD could induce thrombosis in the tumor area, thereby increasing the accumulation of P-aPD-1 in the tumor area. Treatment with tTF-RGD+P-aPD-1 effectively inhibited the growth of CT26 colon tumors and prolonged the survival time of tumor-bearing mice. Flow analysis suggested a significant increase in the number of CD3+ CD8+ T cells and the ratio of IFN-γ+ CD8+ T cells after treatment, confirming that this strategy improved the effect of immune checkpoint inhibitors on T cell activity recovery. In addition, this strategy improved the therapeutic effect of aPD-1 in a 4T1 in situ breast cancer model.
To expand the application of this strategy, the team further explored the responsiveness of platelet derivatives-platelet membrane-encapsulated nanoparticles (PM-NP) to tTF-RGD-mediated coagulation signals. Cell membranes retain many surface receptors from their parent cells, so cell membrane-encapsulated nanoparticles may have similar responsive properties. The researchers loaded the chemotherapeutic drug paclitaxel (PTX) into PM-NP to form PM-NP/PTX and investigated its responsiveness to tTF-RGD-triggered coagulation signals. The team tested the effect of tTF-RGD on tumor accumulation and anti-tumor efficacy of PM-NP/PTX in a patient-derived xenograft breast cancer model. The results showed that tTF-RGD effectively increased the accumulation of PM-NP/PTX in the tumor site, resulting in increased local drug concentration in the tumor, thus improving the tumor suppressive effect of PTX chemotherapy, with great clinical translation potential.
In summary, the researchers used a fusion protein composed of tTF-RGD to initiate a coagulation cascade reaction in the tumor site mimicking physiological signals, which could recruit anti PD-1 antibody-modified platelets (P-aPD-1) and platelet membrane-encapsulated nanoparticles to the tumor region to enhance the effectiveness of immunotherapy and chemotherapy. This strategy artificially creates a platelet-targeting environment that enhances tumor accumulation of platelet-based delivery systems, and is adapted to multiple therapeutic approaches, promising to overcome the limitations of traditional active targeting strategies.
Reference
1. Wang, Yixin, et al. “Active recruitment of anti–PD-1–conjugated platelets through tumor-selective thrombosis for enhanced anticancer immunotherapy.” Science Advances 9.13 (2023): eadf6854.
