Immunotherapy is making significant advancements in oncology treatment. In 2021 alone, the FDA approved over a hundred monoclonal antibody drugs. Alongside monospecific antibodies, the development of multispecific antibodies is also in full swing. Currently, most binary or multiple antibody drugs are in the preclinical or clinical stage, with only a few on the market and three multispecific antibodies available.
Multispecific antibodies can simultaneously bind to two or more different epitopes or antigens, thereby improving antibody targeting and enhancing the killing effect on tumor cells. Based on current clinical results, bispecific antibodies have demonstrated superior outcomes compared to monoclonal antibodies, holding promise as second-generation antibody therapeutics.
Recently, the journal Theranostics published a review article titled “When three is not a crowd: trispecific antibodies for enhanced cancer immunotherapy,” which discusses the basic principles of multispecific antibody design, focusing on their impact on cellular splice apparatuses such as T cells, NK cells, and multiple receptors. It also provides a comprehensive analysis of the ongoing development of multi-specific antibodies under development.
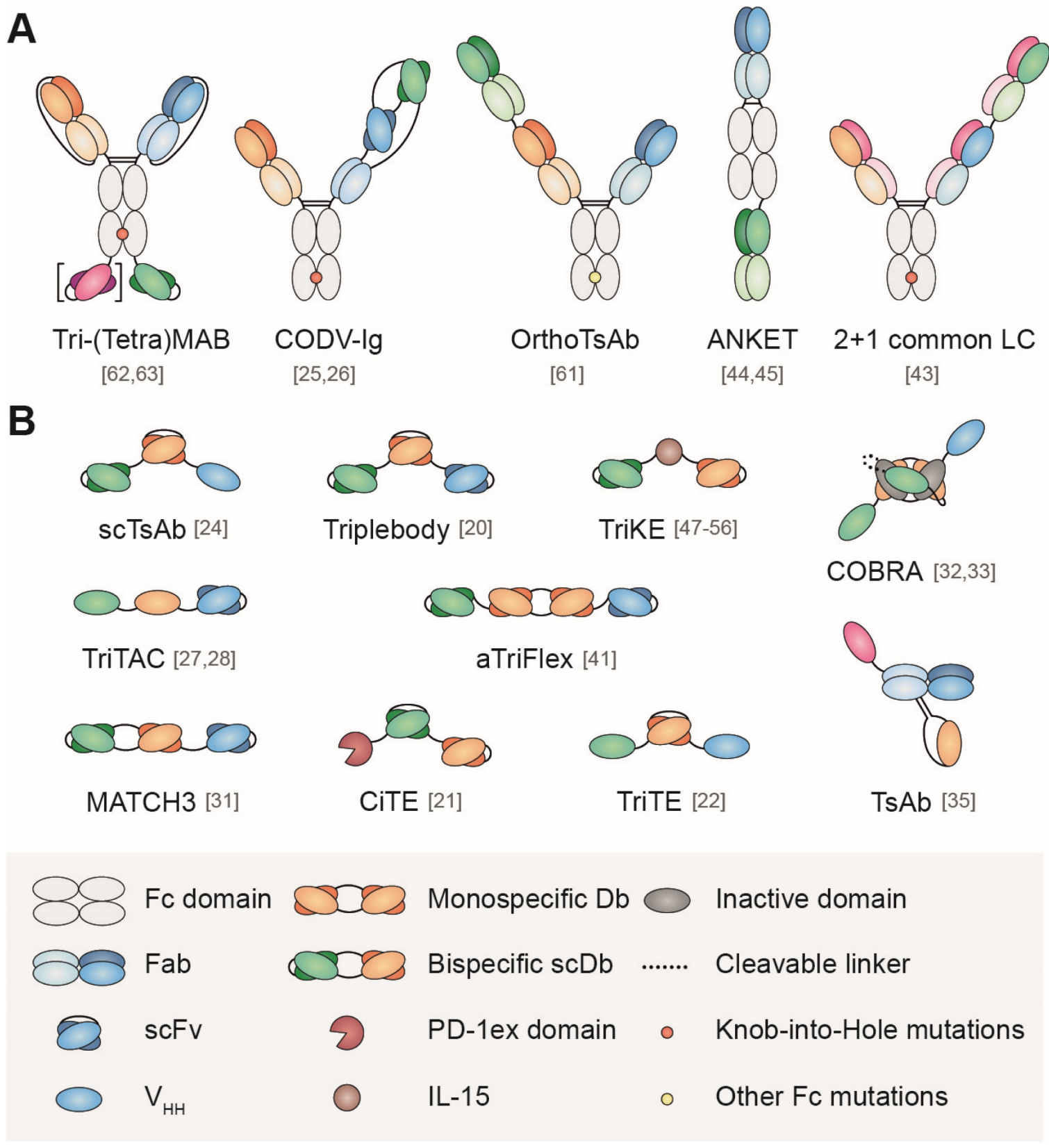
Fig. 1 Schematic representation of some formats of multispecific antibodies with (A) or without (B) Fc domain (Tapia-Galisteo, 2023)
Immune cell engagers are molecules capable of redirecting immune effector cells to target cancer cells, aiming to trigger effective tumor cell killing and establishing a connection between immune cells and target cells. For example, T cell redirection against CD3 enhances tumor specificity and immune cell activation. Dual targeting of tumor-associated antigens (TAAs) may help prevent tumor escape caused by selective pressure and overcome antigen heterogeneity.
Another class of multispecific antibodies recognizes different antigens on the same cell. For instance, Amivantamab recognizes EGFR and cMet, blocking their respective signaling pathways. Tri-specific antibodies (TsAbs) targeting HER2/EGFR/cMet demonstrate robust trispecific binding, but their therapeutic potential remains to be tested.
Current preclinical and early clinical data suggest that multispecific antibodies offer significant advantages in enhancing immune cell potency against intra-tumor heterogeneity and unfavorable tumor microenvironments. However, certain structures of multispecific antibodies may be prone to stability effects, leading to breakage and aggregation. In addition, the production of multispecific antibodies may generate unintended by-products. Therefore, optimization of multispecific antibody development strategies is necessary to address these concerns.
Reference
1. Tapia-Galisteo, Antonio, et al. “When three is not a crowd: trispecific antibodies for enhanced cancer immunotherapy.” Theranostics 13.3 (2023): 1028.
