As we all know, CD19 is the most frequently used target marker for CAR-T cell therapy clinical trials. But not much people knows about the second frequently used target biomarker is mesothelin. What’s more, mesothelin is currently the preferred marker in researches of CAR-T therapy for solid tumor treatment. There are 4.5% of the total number of the world CAR-T clinical trial, if only counted the solid tumor trial, this ratio would rise to 14.5%. What is mesothelin? Why it becomes the most frequently used CAR-T biomarker for solid tumor? Here we can find an answer.
1. Basic knowledge about mesothelin
Mesothelin (MSLN) was first identified in Ira Pastan’s Lab at National Cancer Institute of NIH over 20 years ago in 1992. Ira and his partners reported an antibody reacting with ovarian cancer named mAb K1 which reactivity is limited to the mesothelial cells of the pleura, peritoneum, and pericardium, as well as cells of the fallopian tubes and tonsils. To identify the protein reacting with mAb K1, proteins on the cell surface were labeled with 125I and the cells were treated with phospholipase C to release surface proteins. The results show that the K1 antibody recognized a protein with a molecular weight of 40 kDa on both OVCAR3 and Hela cells. because the protein was expressed in normal mesothelial cells, it was named as mesothelin. Mesothelin is a glycoprotein anchored to the plasma membrane by a glycophosphatidyl inositol (GPI) domain. It is initially synthesized as a 69 kDa cell-surface protein. After cleavage of the amino terminus by the furin protease, a 40-kDa C-terminal fragment remains attached to the membrane and a soluble 32-kDa N-terminal fragment, named megakaryotic-potentiating factor (MPF), is released. soluble form of MSLN has also been detected in the sera of patients with solid tumors, which is referred to as soluble MSLN-related protein (SMRP). SMRP is generated either by alternative splicing or by proteolytic cleavage of the MSLN mature form.
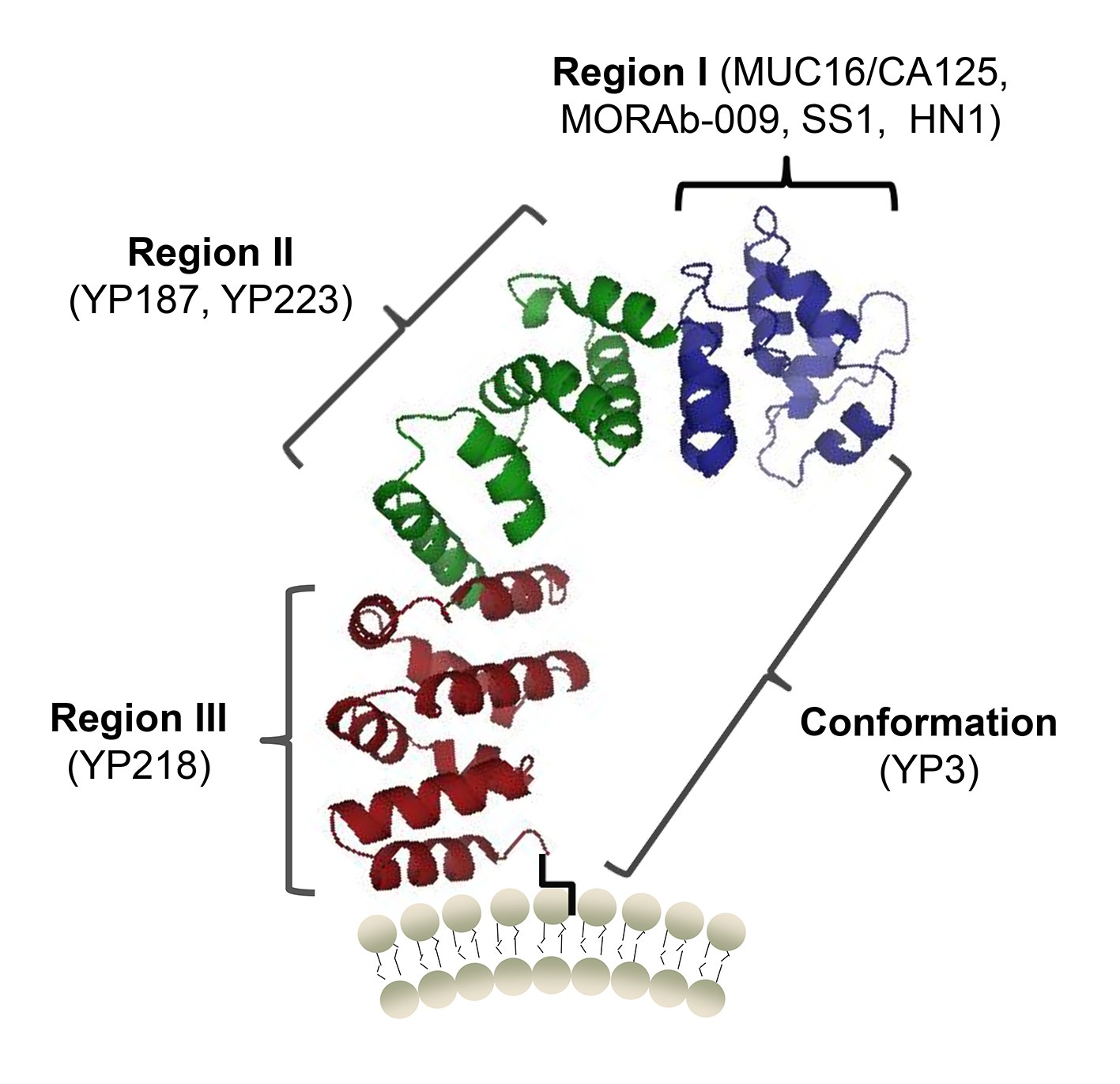
Figure 1. A protein structure model of human mesothelin and the binding sites of MUC16 (CA125) and antibodies (Zhang, 2015)
2. Physiological function of mesothelin
The biologic function of MSLN seems to be nonessential in normal tissues, given that MSLN knockout mice exhibit normal development, reproduction, and blood cell count. MSLN can act bidirectionally, either by directly activating intracellular pathways via its GPI domain or by interacting with its receptor, CA125/MUC16. Overexpression of MSLN alone is sufficient to constitutively activate the NFκB, MAPK, and PI3K intracellular pathways promoting cell proliferation and resistance to apoptosis. MSLN overexpression promotes cell migration and invasion by inducing activation and expression of the matrix metalloproteases MMP7 and MMP9. MSLN expression as well as elevated serum soluble MSLN-related protein levels, is associated with progressing tumor burden, increasing stage, and poor overall survival. That’s why MSLN as a non-essential protein but play an important role in tumor diagnostic and therapy.
3. Mesothelin as diagnostic marker
The differential expression of mesothelin at the cell surface of some cancer cells and in patient fluids makes it suitable as a cancer marker. Mesothelin levels are elevated in about 50% of patients with mesothelioma and ovarian cancer. MPF levels, like mesothelin levels, are elevated in many patients with mesothelioma and ovarian cancer but are not elevated in patients with pancreatic adenocarcinoma.
4. Mesothelin as immunotherapy marker
Mesothelin is an excellent target for antibody-based therapies and encouraged the development of agents that could be used in patients. The first mesothelin-directed agent to enter the clinic was the immunotoxin SS1P, it contains a bacterial protein and the consideration of the safety of SS1P should be taken. MORAb-009 (amatuximab) is a chimeric antibody directed to mesothelin. In preclinical studies, it kills mesothelin-expressing cell lines by antibody-dependent cell-mediated cytotoxicity and also inhibits the interaction between mesothelin and CA-125. CRS-207 is the only mesothelin tumor vaccine currently in clinical development which consists of a live-attenuated strain of the bacterium Listeria monocytogenes expressing human mesothelin. As mesothelin is initially identified by monoclonal antibody. That makes it a natural target of chimeric antigen receptor T cells. A particular concern regarding MSLN CARs is interference from soluble MSLN, which in principle could occupy and block the scFv portion. Reassuringly, MSLN CAR T-cell activation (cytokine secretion and cytotoxic activity) is dependent on MSLN expression on the cell surface. Significantly, the presence of serum SMRP does not alter MSLN CAR T-cell efficiency in vitro or in vivo, even at high levels. The lack of CAR blockade by serum protein may be explained by the avidity of CAR T cells for membranous target antigen, which may be increased by interactions between adhesion molecules and other accessory molecules present on the surface of the T cell and tumor cells.
The solid-tumor microenvironment poses several obstacles for mesothelin CAR T cells that may limit their antitumor efficacy. To optimize the efficiency of CAR T cells, numerous approaches are under evaluation to tame the host tumor microenvironment or generate “armored” CAR T cells that can overcome immune barriers. Such strategies include
- promoting CAR T-cell infiltration;
- augmenting the functional persistence of CAR T cells;
- enhancing CAR T cells to overcome inhibitory signals encountered in the tumor microenvironment;
- improving safety by preventing on-target/off-tumor toxicity
Clinical trials of different agents targeting mesothelin have confirmed that it is an excellent target for cancer immunotherapy, and much of the effort is now focused on larger trials to confirm the preliminary hints of antitumor activity seen in patients. CAR therapy using second-generation CARs has rapidly translated to clinical impact in CD19+ malignancies, paving the way for unprecedented enthusiasm for adoptive cell therapy and engineered T cells. Having such a powerful technology at hand, one important future direction for CAR research is the identification of suitable targets for tackling solid tumors. MSLN offers exciting prospects based on its high expression in a variety of cancers and low level expression in normal tissues. The latter commands a thoughtful targeting strategy, noting that mesothelin-targeted immunotherapies have been very well tolerated. These clinical outcomes, combined with the preclinical data obtained with MSLN CARs, argue favorably for clinical trials targeting mesothelioma and breast, lung, ovarian, and pancreatic cancers, which will soon be performed at multiple centers.
Reference
Zhang, Yi-Fan, et al. “New high affinity monoclonal antibodies recognize non-overlapping epitopes on mesothelin for monitoring and treating mesothelioma.” Scientific reports 5.1 (2015): 9928.
