As the data from clinicaltrials.gov shows, CD19 antigen is the most frequently used biomarker in CAR-T cell therapy clinical trials for hematological malignancy such as leukemia and lymphoma. According to our statistics report, over 300 completed and ongoing CAR-T clinical trials registered all over the world, among which about 150 are targeting on CD19, count for 50% of the total number. CD19 is obviously the most important molecular biomarkers for CAR-T immunotherapy for now.
CD19 is so important, do you really know about CD19 antigen? What is a CD19 antigen?
1. Basic knowledge about CD19 molecule
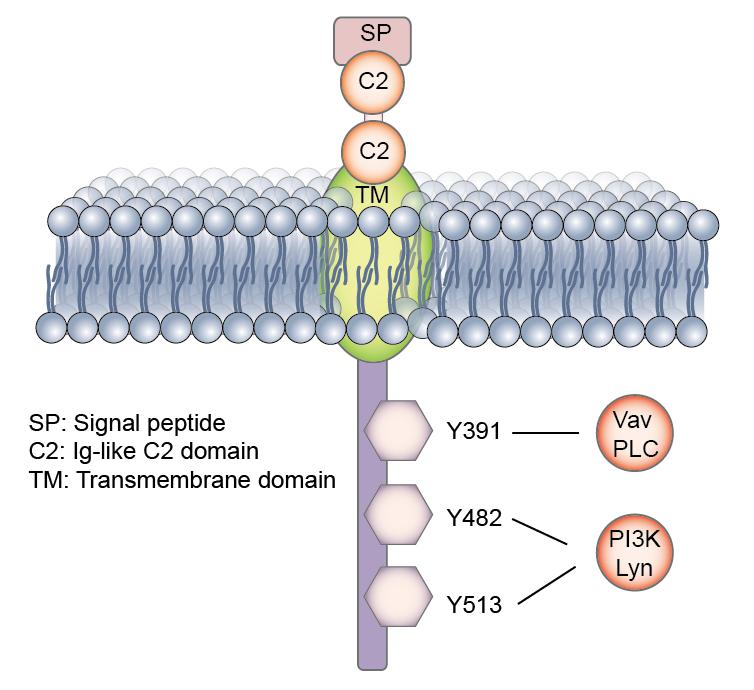
CD19 is first identified as B4 antigen of human B lymphocytes through the use of B4 monoclonal antibody on 1983 by Lee M. Nadler and his colleagues. B4 can be detected on all normal, mitogen-stimulated, and malignant B cells, excluding the plasma cell. Then, cDNA encoding CD19 of human and mouse B lymphocytes was isolated and identified. The human CD19 antigen is a 95 kd transmembrane glycoprotein belonging to the immunoglobulin (Ig) superfamily. It is encoded by the 7.41 kilobite cd19 gene located on the short arm of chromosome 16, 16p11.2. The gene contains 15 exons and codes for the CD19 molecule with 556 amino acids (Figure 1).
CD19 is classified as a type I transmembrane protein, with a single transmembrane domain, a cytoplasmic C-terminus, and extracellular N-terminus. No significant homology exists between CD19 and other known proteins. The extracellular element contains two C2-type Ig-like domains divided by a smaller potential disulfide linked non-Ig-like domain, as well as N-linked carbohydrate addition sites.
CD19 is specifically expressed in normal and neoplastic B cells, as well as follicular dendritic cells. During B cell lymphopoiesis, the surface expression of CD19 first takes place around the time of immunoglobulin gene rearrangement. The surface density of CD19 is highly regulated throughout B cell development and maturation until the loss of expression during terminal plasma cell differentiation.
CD19 is one of the most reliable surface biomarker for B lymphocytes. Its expression in mature B cell are 3-fold higher than that in immature B cells, with slightly higher expression in B1 cells than in B2 cells. it is expressed from pre-B cell until the terminal differentiation to plasma cells.
2. Physiological function of CD19
CD19 is critically involved in establishing intrinsic B cell signaling thresholds through modulating both B cell receptor (BCR)-dependent and independent signaling. It plays roles in the antigen-independent development as well as the immunoglobulin-induced activation of B cells. CD19 is thus critical for the body to mount an optimal immune response. CD19 works in complex with the BCR and other surface molecules to allow both direct and indirect recruitment and binding of various down-stream protein kinases, such as Lyn, Fyn from Src family, the Ras family, adapter molecules includes Vav, Grb2, and PI3K.
CD19 functions as the dominant signaling component of a multimolecular complex on the surface of mature B cells, alongside complement receptor CD21, and the teraspanin membrane protein CD81 as well as CD225. CD19 acts as a critical co-receptor for BCR signal transduction. It recruits and amplifies the activation of Src-family protein tyrosine kinases such as Lyn and Fyn. Upon BCR activation, CD19 also enhances BCR-induced signaling crucial for B cell expansion, through recruitment and activation of PI3K and downstream Akt kinases. CD19 is thought to play dual roles in B cell activation. First, it functions as an adaptor protein to recruit cytoplasmic signaling proteins to the membrane. The second role of CD19 is as a signal subunit for the CD19/CD21 complex when colligated with the BCR, where Ag bearing complement enhances B-cell activation via BCR-CD19/CD21 coligation.
CD19 overexpression is associated with a dramatic reduction (>80%) in the number of peripheral B cells, primarily due to impaired generation and early development f immature precursors in the bone marrow. CD19 deficiency mice show dramatic reductions in the total number and frequency of peripheral and splenic B cells, especially of B1 cells, a B cell subtype found in the peritoneal cavity that normally express higher IgM levels than conventional B (B2) cells, and low levels of IgD.
3. CD19 and diseases
As CD19 shows essential role in B cell development and maturation. It is observed and proved that associated withnumerus human diseases. CD19-deficient humans and mice exhibithypo responsiveness to transmembrane signals, and weak Tcell dependent humoral responses, leading to an overall impaired humoral immune response. There ishypothesis that CD19 may play an important role in in vivo modulation of MHC class II expression and signaling. Such discoveries have led tomounting interest in CD19 as a potential immunotherapy target for various autoimmune disorders, including rheumatoid arthritis and multiple sclerosis.CD19 expression is highly conservedon most B cell tumors. It is expressed in most acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL) and B cell lymphomas. In fact, the majority of B cell malignancies express CD19 at normal to high levels (80% of ALL, 88% of B cell lymphomas and 100% of B cell leukemia). CD19 levels can potentially be useful as a diagnostic tool in distinguishing certain lymphoma subtypes. Follicular lymphoma, for example, has lower CD19 level more frequently than any other lymphoma subtypes.
4. Anti-CD19 therapy
According to the physiological function in B cell signaling and its diagnostic use in many hematologic malignancies, CD19 have been treated as a potential therapeutic target by some monoclonal antibodies for lymphoma and leukemia therapy. Novel immunotherapy approaches such as bispecific antibodies, ADCs, Fc-engineered antibodies and chimeric-antigen receptor (CAR)-transduced T cells are also designed to target CD19 and have already generated promising results in clinical trials. CD19 antibody-based therapy has the potential to become a valuable addition to the armamentarium of hematology drugs.
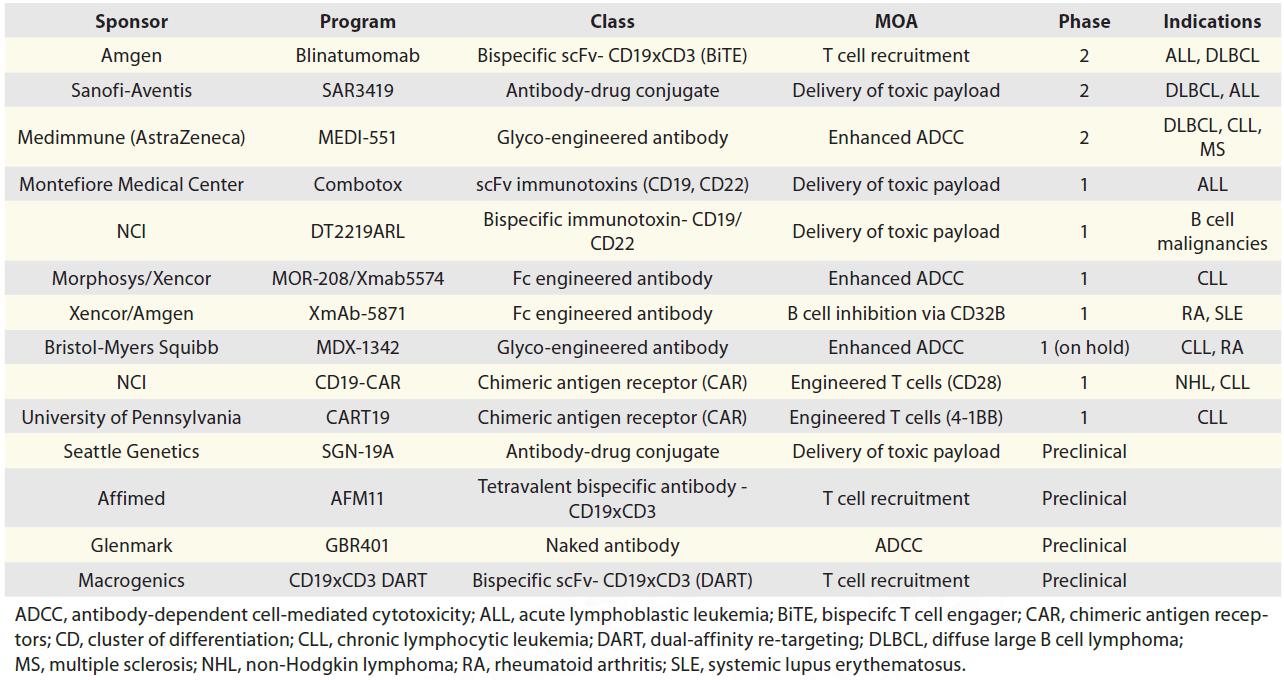
Here we introduce some of these antibody based CD19 Programs in development.
Blinatumomab (Amgen):
Blinatumomab is currently the most advanced CD19 program, with two pivotal Phase 2 studies in progress. It belongs to a novel class of bispecific antibodies, bispecific T-cell engager (BiTE), that redirect T cells to attack cancer cells. It comprises two scFvs that bind CD3 and CD19, respectively. Upon simultaneous binding of both targets, blinatumomab brings a T cell and a target cell in close proximity, which leads to T cell activation and subsequent killing of the target cell.
SAR3419 (Sanofi/Immunogen):
SAR3419 is an anti-CD19 ADC currently in Phase 2 studies. The molecule is composed of the humanized antibody huB4 conjugated to the maytansine derivative DM4 via a cleavable disulfide linker. Upon binding to CD19 on the surface of cells, SAR3419 is internalized and delivers its toxic payload into the cell, resulting in inhibition of microtubule assembly and cell death.
MEDI-551 (AstraZeneca):
MEDI-551 is an Fc-engineered humanized CD19 antibody with enhanced antibody-dependent cell-mediated cytotoxicity (ADCC). Because the antibody is produced in a fucosyltransferase-deficient cell line, it is afucosylated and therefore has increased binding to FcγRIIIA.
Combotox (Montefiore Medical Center):
Combotox is a mixture of two immunotoxins that target CD19 and CD22, respectively. Both immunotoxins are scFv antibodies fused to deglycosylated ricin A chain. Combotox and each immunotoxin demonstrated potent activity in in vitro and in vivo models of B cell leukemia.
Others are listed in the following tables.
5. CD19-targeted chimeric-antigen receptors
Chimeric antigen receptors (CARs) are T cells that are genetically modified to express a targeting moiety (most commonly antibodies) on their surface. These targeting moieties confer the desired specificity toward cells that express a given target, regardless of T cell receptor (TCR) specificity. Typically, the antibody is fused with an intracellular signaling domain of the TCR complex (CD3-zeta chain). Upon binding target cells via the antibody moiety, CARs undergo activation that leads to proliferation, cytokine production, and lysis of target cells. Anti-CD19-CAR is generated by retroviral transduction of an anti-CD19 antibody fused to CD3 zeta chain and CD28 endodomain as a co-stimulatory factor.
The enthusiasm of many groups for targeting CD19 with CARs is testimony to the attractive features of CD19 and its broad relevance as a CAR target. Five major lessons have come out of the last two decades of CD19 CAR research:
a. Cells work where drugs have failed.
The most dramatic lesson learned from the CD19 paradigm is that engineered T cells induce complete remissions, including molecular remissions, in subjects for whom chemotherapies, often utilizing multiple drug combinations, have led to drug resistance and tumor progression.
b. Academic T cell manufacturing is robust and dependable
A handful of academic centers have successfully established manufacturing procedures that have proven to be reproducible and dependable.
c. Next-generation CARs are potent.
The successes we have seen in CD19 CAR therapy were all obtained with second-generation CARs, supporting the critical importance of incorporating costimulatory signals in recombinant antigen receptors.
d. CAR T cells may induce toxicities
The CD19 paradigm has revealed three potential toxicities of CAR therapy: B cell aplasia, severe cytokine release syndrome (CRS), and neurological toxicity.
e. Antigen escape may still occur.
CAR T cells most often target a single antigen. One must therefore seek to identify target antigens that, ideally, are present in all tumor cells, including cancer stem cells, if possible. A broad effort is now underway to identify suitable targets for a variety of cancers. It is expected that the selective pressure imparted by CAR T cells, as for any targeted therapy, will sometimes yield antigen escape variants.
CD19 CARs have taught us a great deal about the enormous potential and current limitations of CAR technology, providing insights into how to tackle solid tumors, which is one of the major next steps for the CAR field.
Structure, function, disease association and antibody based therapy including CAR-T therapy, those above are everything we know about CD19 antigen.
Creative Biolabs is a world-renowned service provider for immunotherapy. We have one of the world’s largest collection of CAR products of different generations targeting various biomarkers, and we continue to innovate the next generation CAR technologies to achieve even greater results. Based on advanced technology and years of research, Creative Biolabs offers high-quality custom service covering the entire CAR-T therapy development process to best suit your technical, program and budget requirements which can greatly assist your research, preclinical investigation and clinical stage development. To see more information about CD19 CAR-T therapy and product, please visit our website at https://www.creative-biolabs.com/car-t/.