The detection and destruction of cancer cells by immune cells is the basis of all modern immunotherapies, including cancer vaccines, checkpoint blocking, and adoptive immune cell therapy (ACT). To achieve the productionRead More…
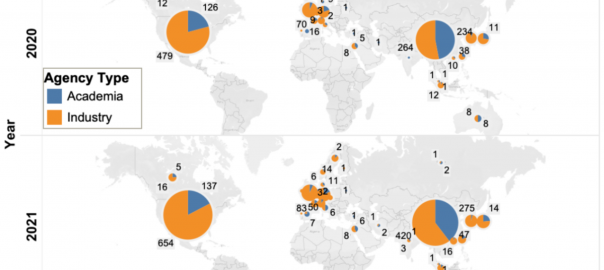
Overview of Global Pipelines of Cell Therapy for Cancer
In this March, Bristol-Myers Squibb and Bluebird Bio announced that their CAR-T cell therapy idecabtagene vicleucel (Abecma) targeting B cell maturation antigen (BCMA) has been approved for the treatment of multiple myeloma,Read More…
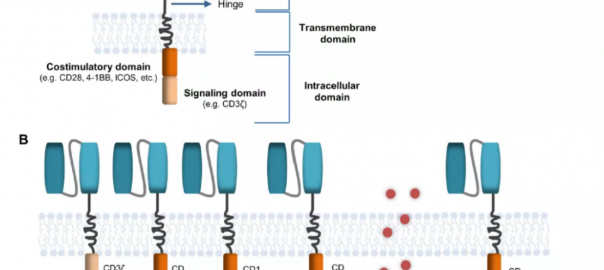
Talking About the New Targets and Techniques of CAR-T Therapy
Adoptive immunotherapy is a transformational therapy in hematology. One of the most representative adoptive immunotherapies is chimeric antigen receptor (CAR) T cell therapy. It expresses and synthesizes CAR on T cells byRead More…
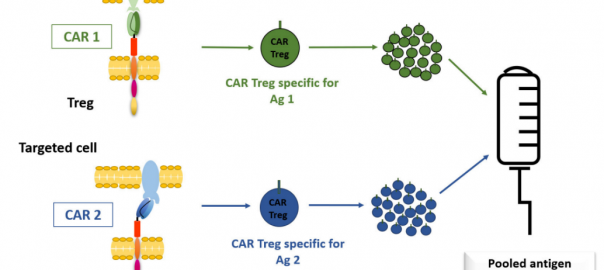
The Future of Regulatory T Cell (Treg) Therapy: CAR-Treg
Regulatory T cells (Tregs) is a small group of immune cells, which specifically inhibit excessive immune activation and maintain immune homeostasis. In the past two decades, the advances in genome editing andRead More…
The U.S. FDA Approved the First BCMA-targeted CAR-T Therapy
Bristol-Myers Squibb (BMS) and bluebird bio announced that the Food and Drug Administration (FDA) has approved the marketing of Abecma (idecabtagene vicleucel), a chimeric antigen receptor T (CAR-T) cell immunotherapy targeting BRead More…
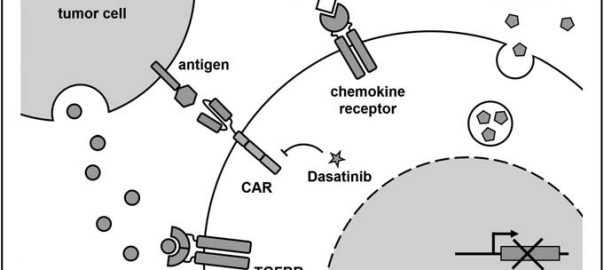
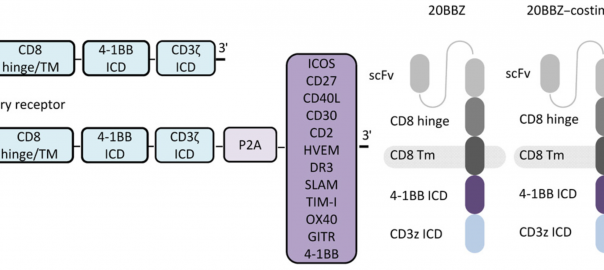
Science Translational Medicine: New Costimulatory Signal Enables CAR-T to Show Potential of Treating Solid Tumor
T cells expressing chimeric antigen receptors (CAR-T) are one of the major breakthroughs in the field of cancer treatment in recent years, and they have achieved excellent efficacy in the treatment ofRead More…
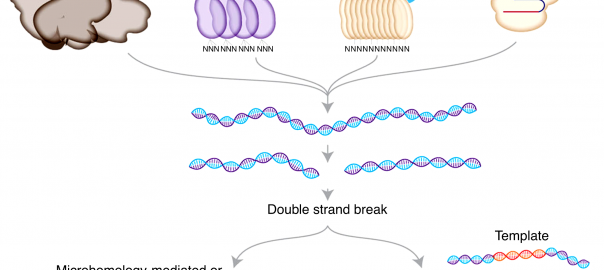
CAR-T Therapy Evolution—Application of Non-viral Vector and Gene Knock-in Technology
As a precision targeted therapy for tumor treatment, CAR-T (chimeric antigen receptor t-cell immunotherapy) has achieved promising results in clinical tumor treatment with optimization and improvement in recent years. It is cutting-edgeRead More…
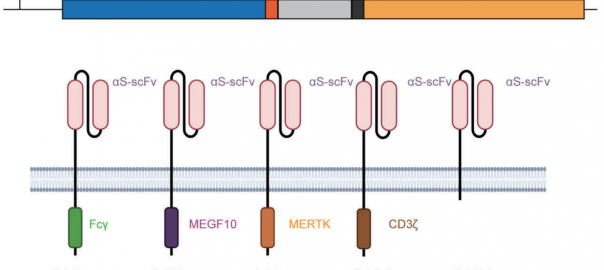
The Potential of CAR-Macrophages Therapy in Novel Coronavirus Clearance
At present, the vaccine research for the Coronavirus Disease (COVID-19) is in progress, but the drug research and development for severe COVID-19 remains a thorny scientific problem. Up to now, there isRead More…
FDA Approves the First IND Application for Universal CAR-T Therapy Derived from iPSC
On July 9th, Fate Therapeutics announced that U.S. Food and Drug Administration has approved the first IND application for iPSC-derived allogeneic CAR-T cell therapy FT819 for the treatment of recurrent/refractory B-cell malignant tumors,Read More…
HLA System and HLA Typing
The human leukocyte antigen (HLA) system or complex is a group of highly polymorphic genomes on chromosomes that encode major histocompatibility complex (MHC) proteins which are expressed on the surface of cellRead More…