CAR-T, Chimeric Antigen Receptor T-Cell Immunotherapy, is the most effective treatment of malignant tumors. Similar to other immunotherapy, its basic principle is to remove cancer cells in the use of the patient’s own immune cells, but the difference is that this is a cell therapy instead of a drug.
Signal
The T cell activation signal area is still the bone of contention in the CAR T establishment.
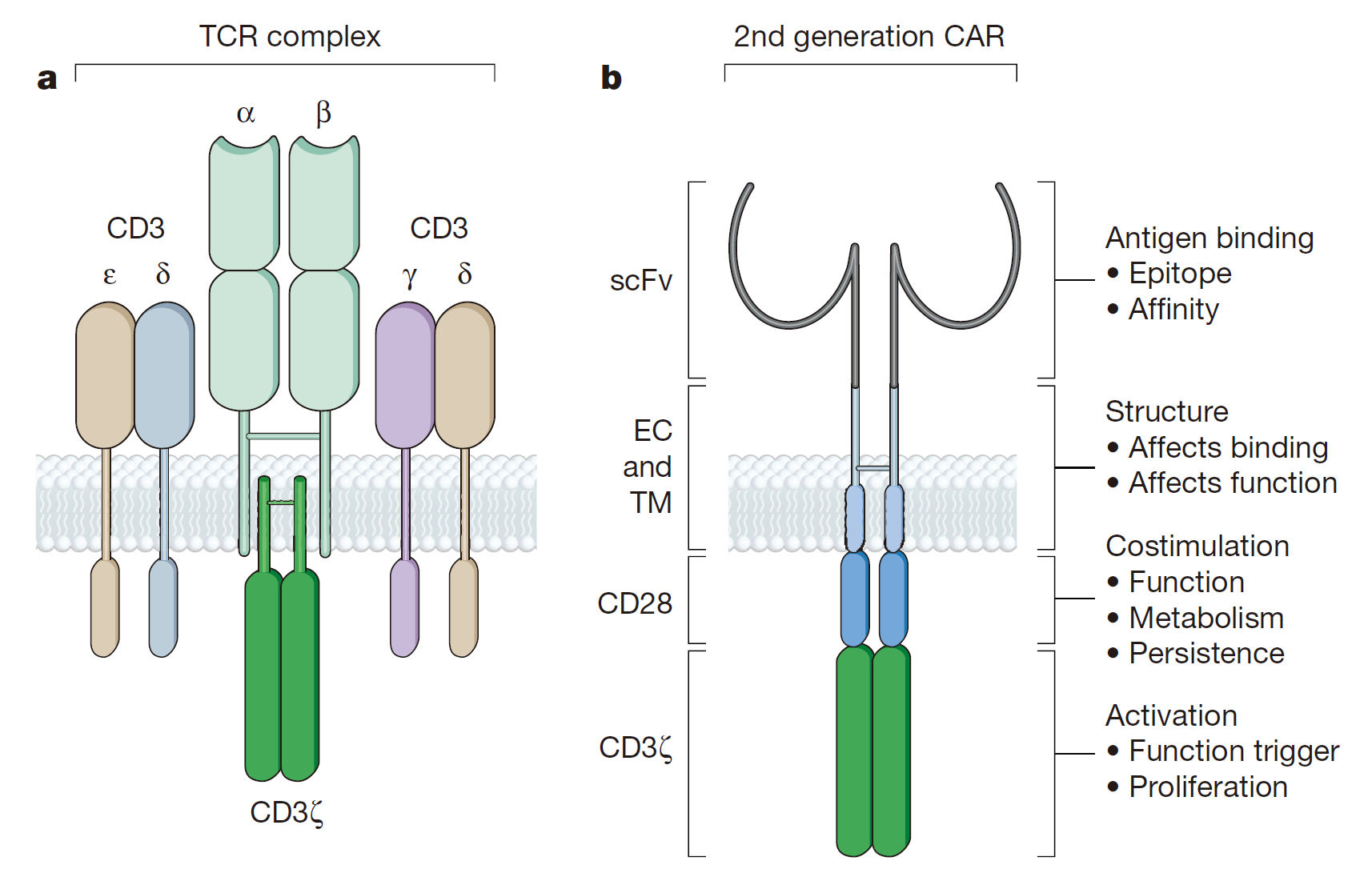
The activation of the first generation of CAR-mediated T cell is accomplished by the tyrosine-activated motif on the CD3z chain or FceRIg. CD3z chains can provide ‘signal 1’ for T cell activation, cleavage of target cells, regulation of IL-2 secretion and antitumor activity expression in vivo. It is reported that the anti-tumor activity of the first generation of CAR-modified T cells has been limited in vivo, and the anti-tumor potential of nitrogen has been confirmed by preclinical experiments. Only the “signal 1” T cell reaction is incompetent with the reduction of cytokine secretion and proliferation, eventually leading to the apoptosis of T cell. The removal of tumor cells also requires a co-stimulatory signal on the surface of tumor cells.
In order to strengthen the CAR activation signal and break through the limitations of the first generation CAR, the second generation CAR combined the stimulus structure has been developed. CD28, the first T-cell costimulatory signal receptor, can bind to the B7 family members on the surface of the target cell. Based on the current T cell activation model, CD28 provides a second stimulus signal (signal 2) activated by T cell to expand “signal 1 ”from TCR/CD3 complex. The costimulation of CD28 can promote the proliferation of T cells, the synthesis and expression of IL-2 and the secretion of anti-apoptotic protein Bcl-xL. To simulate the activation of this T cell, the second generation of the CAR design also includes the “signal 1” of the CD3z chain and the “signal 2” of the CD28. Many studies have shown that compared the 1st generation, the 2nd generation of CAR with the “second signal” remains the same level of antigen specificity with the increment of T cell proliferation, cytokine secretion, anti-apoptotic protein secretion and the delay of cell death. Moreover, the CD28 in CAR can also help transformed T cells to counteract the adverse effects of tumor cell microenvironment.
Different costimulatory molecules play a significant role in the development of the 2nd generation of CAR. CD3z signals are combined with other B7 family members or family members of tumor necrosis factor receptor. CD28 and CD137(4-1BB) are two alternative CARs for different tumors in vivo and vitro. In addition, CD244, an NK cell receptor, is used to increase the memory effect on tumor cell lysis and the CAR-mediated lethal effect.
To further improve the design of CAR T therapy, many research groups began to focus on the development of the 3rd generation CAR, including “signal 1”, “signal 2” and additional costimulatory signals. The results of the second-generation CAR and third-generation CARs obtained by different researchers using different materials are contradictory. The direct comparison between the second generation and the third generation CAR has not yet been carried out, so what kind of design is better between the two is not yet conclusive. However, the current second and third generation of design have their own clinical trial applications.
Migration
The targeting T cells for genetically engineered tumor cells must migrate to the corresponding sites to eradicate the disease. It has been proved for many times that CAR-T cells can migrate to the periphery of the tumor and recent clinical trials provide more evidence for this: GM-CAR-modified T cells are injected into the patient and CAR can migrate to different tissues. Although CAR-T migration has been experimentally verified, it still remains unclear whether T cell genetic modification and in vitro amplification will affect necessary chemokines or cytokines for migration. One of the strategies to promote T cell migration is to transform genes from the expression of cytokine receptors. Many studies have implemented this principle by expressing CXCR2 (CXCL1 receptor) and CCR4 (CCL17 receptor) in CAR-T cells. In addition to this strategy, finding other methods of migrating T cells to tumor sites is critical to the successful elimination of tumors.
Survival
In order to successfully eliminate the tumor, the targeted T cells must be able to exist in the body for a long enough time. Cell Immunology studies using tumor lymphocytes have found that there is a positive correlation between the persistence of adoptive T cells and clinical efficacy. Regulatory chemotherapy can prolong the presence of adoptive T cells through a variety of ways. Patients who initially received CD19-targeted T-cell therapy did not have a combined regimen, which made the presence of subsequent T cells in the body almost impossible to detect. And when the patient received a combination of mediating chemotherapy treatment, the genetically modified T cells can still be found after 6 weeks of immunization in the patient’s bone marrow.
Reference
1. Sadelain, Michel, Isabelle Rivière, and Stanley Riddell. “Therapeutic T cell engineering.” Nature 545.7655 (2017): 423-431.