Myasthenia gravis (MG) is an autoimmune disease characterized by an acquired neuromuscular junction transmission disorder mediated by autoantibodies. MG patients are mainly characterized by fluctuating weakness and fatigue of skeletal muscles, with extraocular muscles frequently involved first, which can involve the whole body. Autoantibodies are of great value for elucidating the pathogenesis and diagnosis of MG. We currently have a good understanding of the autoantibodies produced by MG patients. The pathological mechanism of complement is also deserving of attention and comprehension for the large percentage of acetylcholine receptor (AChR) antibody-positive MG patients.
Introduction to MG
MG has been known for a long time due to its uncommon occurrence but obvious clinical symptoms, and it was first described as an ancient rare disease by Thomas Willis in the book in 1672. From the initial symptomological description to the gradual in-depth study of its pathological mechanism, people’s understanding of MG continues to deepen as medicine advances. The most prevalent pathogenic antibodies in MG are now known to be AChR antibodies, which were first discovered and described in the 1970s. The discovery of the AChR antibody is regarded as a turning point in the study of MG since it raises people’s understanding of the disease to the level of molecular biology, which is crucial for MG diagnosis and treatment. Later, the researchers found other potential autoantibodies in MG patients.
MG classification
According to the characteristics of the serum antibodies and the clinical characteristics of patients, subgroups of MG can be divided into the following six categories, which are helpful for the targeted treatment of MG after diagnosis.
(1) Ophthalmic MG (ocular MG, OMG): The patient’s muscle weakness is confined to the ocular muscles.
(2) AChR-gMG (generalized MG, gMG): The patient’s serum acetylcholine receptor antibody is positive.
(3) MuSK-MG: In about 1%-4% of MG patients, serum muscle-specific receptor tyrosine kinase (MuSK) antibody is positive.
(4) LRP4-MG: low-density lipoprotein receptor-related protein 4 (LRP4) positivity in serum.
(5) Antibody-negative MG: Negative in antibody test mentioned above.
(6) Thymoma-related MG: a paraneoplastic syndrome.
About 85% of patients have antibodies to AChRs, which have been the subject of the most research. Clinically, patients of the AChR-gMG type made up the largest percentage. According to the age of onset, it can be further divided into early-onset (<50 years old) and late-onset (>50 years old), and some patients with this type of OMG may also experience whole-body spread.
AChR antibodies: AChR-gMG-specific antibodies
AChR antibodies are produced by AChR-gMG patients against postsynaptic membrane acetylcholine receptors, which is a class of autoantibodies that are considered specific for AChR-gMG patients. Muscle weakness, a well-known pathological process, is caused by the binding of AChR antibodies to AChR in AChR-gMG patients. Clinically, the detection of AChR antibodies in patients is an essential basis for the diagnosis of AChR-gMG. If you only know about AChR antibodies for AChR-gMG, you only have a small portion of the picture.
AChR antibodies block neuromuscular junction signaling by three mechanisms:
(1) Block the binding of ACh to AChR: AChR antibody binds to the extracellular domain of AChR and may directly bind to the binding site of ACh on AChR, thus hindering the binding of ACh to AChR, which is the easiest one to understand.
(2) Accelerate the internalization and degradation of AChR and reduce the number of AChR: AChR is the main antigen of the posterior membrane at the nerve-muscle junction, and the AChR antibody is a bivalent antibody, which can bind two antigens at the same time and can produce an antigen modulation process, resulting in endocytosis and degradation of AChRs on the surface of muscle cells.
(3) Complement activation: The most critical mechanism by which the AChR antibody binds to AChR to cause muscle weakness is thought to be this one. Complement is an essential mechanism in the pathogenesis of AChR-gMG, and its role in MG has received a lot of attention in recent years.
Complement plays a crucial role in the pathogenesis of AChR-gMG
The complement system is an essential member of the human immune system. Under normal conditions, complement contributes to both innate immunity and humoral immunity. The complement system is complex and precise. More than 30 proteins are involved in the regulation of complement to ensure the “smooth operation” of the complement system, which can both play an immunological role and prevent damage to its normal components.
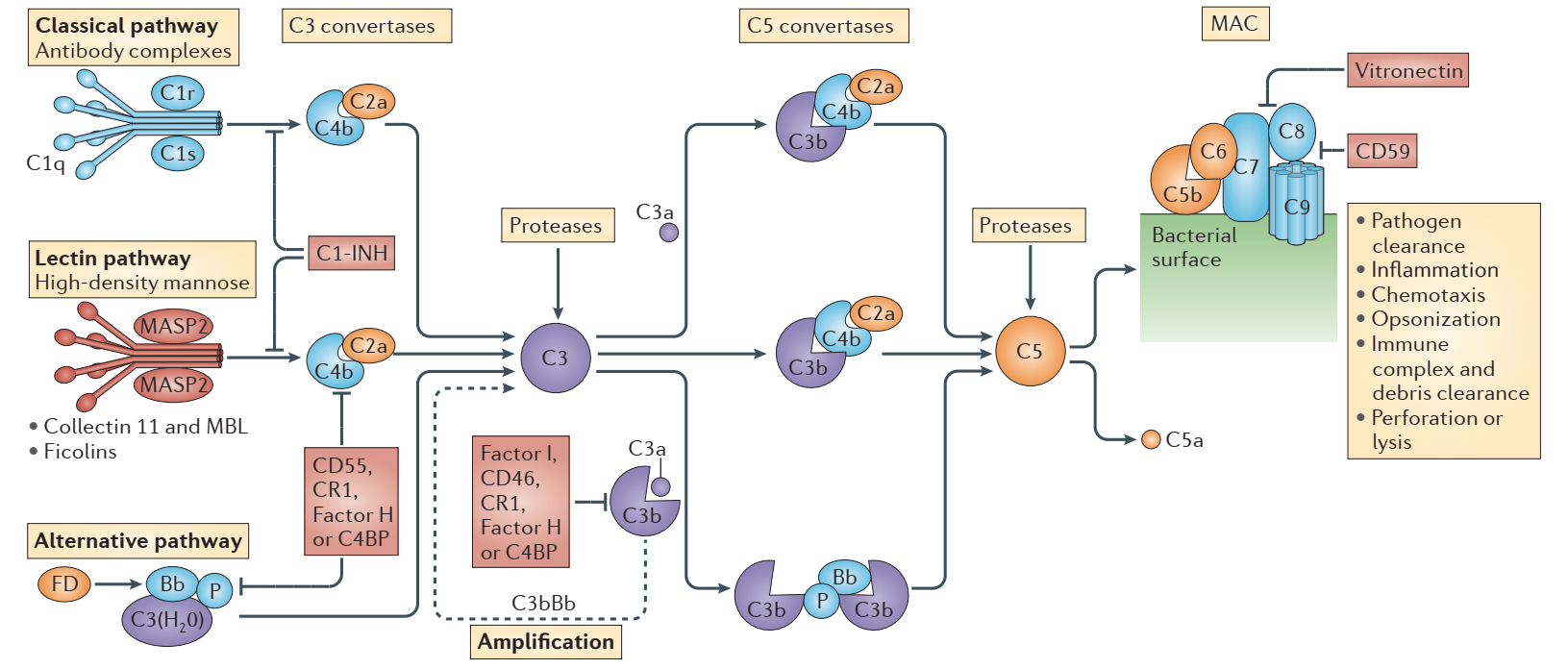
Complement activation pathways include the classical pathway, the alternative pathway, and the lectin pathway. The complement system is activated by the three pathways, and a series of cascade reactions are regulated as a result. It should be pointed out that they share a common terminal pathway: complement factor C5 is split into C5a and C5b, and C5b continues to undergo cascade reactions to form membrane attack complexes (MAC) with multiple units of C6, C7, C8, and C9. Target cells can be lysed by MAC, which also aids in the removal of foreign microorganisms and apoptotic cells from the body. Complement overactivity can cause MAC to attack its cells, thereby resulting in immunological damage and disease.
Disclaimer: Creative Biolabs focuses on promoting biological and biomedical research globally. This article is for information exchange purposes only. This article is also not a treatment plan recommendation. For guidance on treatment options, please visit a regular hospital.
