The alternative pathway is a branch of the complement network, which is generally recognized as an important conservator to recognize and eliminate the pathogens through direct killing or stimulation of phagocytosis. Besides the immunoregulatory functions, complement system has been elaborated on the pathogenic role of ischemic injury, inflammatory, and autoimmune diseases.
The alternative pathway is an independent defense mechanism of the immune response, and it plays an indispensable role in the defense against infections, especially in the early stage while specific antibodies have not been produced. In the physical circumstances, this pathway is slowly activated by spontaneous hydrolysis of the internal C3 thioester bond. When the foreign excitant (e.g. various proteins, lipids and carbohydrate structures on microorganisms) contact with C3, alternative pathway is amplified quickly.
Components of Alternative Pathway
Compared with classical pathway and lectin pathway, the common components are C3, C5-C9, while the unique components are:
-
Factor B
-
Factor D
-
Factor P (properdin)
Aided by factor B/factor D-targeted transgenic mice, investigators demonstrated that all of them are required for efficient alternative pathway activation by zymosan.
Activation Mechanisms of Alternative Pathway
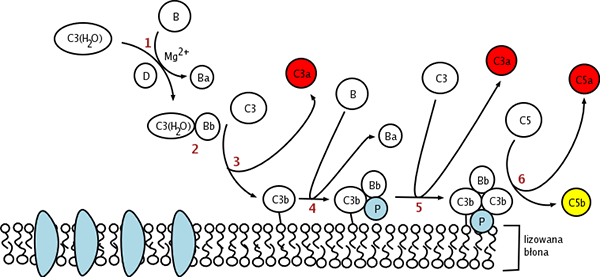
In contrast to classical and lectin pathway, the alternative pathway is capable of autoactivation because of a process termed “tickover” of C3. A conformationally altered C3, designated C3(H2O) is the production of tickover. Normally, the rate of spontaneously tickover is extremely low to 1% of total C3. C3(H2O) has the capability to bind factor B, which can be cleaved into Ba and Bb followed by the constitutively active serum protease factor D. Bb remains bound to C3(H2O) to form C3(H2O)Bb, the alternative pathway C3-convertase, which has the serine protease activity to cleave multiple C3 into C3b to generate more C3 convertase in a powerful amplification loop, resulting in the full activation of the complement system. C3bBb is unstable until it binds properdin to form the complex C3bBbP, a stable form which can bind an additional C3b to form C5-convertase, (C3b)2BbP. Then the alternative pathway follows the same path as the classical or lectin pathway. C5-convertase cleaves C5 into C5a and C5b. C5b recruits C6, C7, C8 C9 to form membrane attack complex (MAC) inducing cell lysis.
 Distributed under CC BY-SA 3.0, from Wiki,
without modification.
Distributed under CC BY-SA 3.0, from Wiki,
without modification.
Fig.1 Diagram depicting the activation processes of the alternative pathway.
The Regulation of Alternative Pathway
Because of the spontaneous activating capabilities, the C3 convertase amplification loop in alternative pathway requires rigorous control to prevent inadvertent inflammation and damage.
-
Complement Factor I (CFI): it is a plasma protease, which prevents the reassembles of the C3 convertase by proteolytic inactivation of C3b to iC3b (inactive C3b).
-
Complement Factor H (CFH): it plays important regulatory functions via several ways. It inhibits the formation of the C3 convertase by competing with factor B for binding to C3b, and accelerates the dissociation of the C3bBb convertase complex. Moreover, it acts as a cofactor in the cleavage of C3b to iC3b.
-
Complement Factor H-Related protein (CFHR): it plays control action by competing with CFH for binding to C3b. CFHR5 is demonstrated as a cofactor for CFI.
Creative Biolabs provides a full range of therapeutic antibodies, inhibitors, soluble complement regulators, as well as customized services based on the alternative pathway. If you are interested, please feel free to contact us for more details.
Published Data
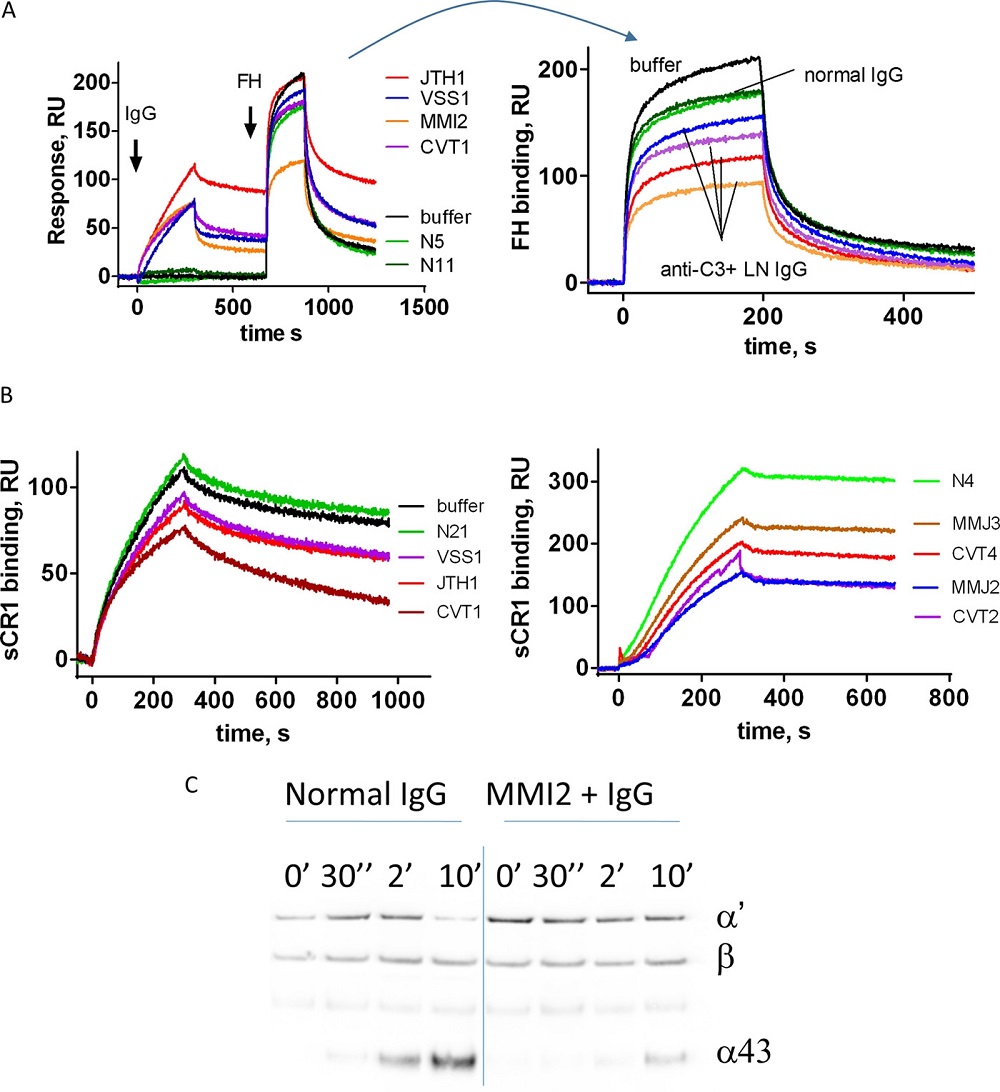 Fig.2 Effects and significance of anti-C3 antibodies.1
Fig.2 Effects and significance of anti-C3 antibodies.1
Lupus nephritis, alias as LN, a sequel of systemic lupus erythematosus, implicates complement component C3, which is essential for the alternative pathway's initiation. In a study, scientists quantified autoantibodies targeting C3 in the plasma of 39 individuals with LN and identified the specific sites on C3 that these autoantibodies recognize. The influence of these IgG autoantibodies from patients on the interaction of C3b with Factor B, Factor H, and complement receptor 1 was examined using surface plasmon resonance. Meanwhile, flow cytometry was employed to analyze the ability of these antibodies to interfere with the formation of C3 convertase on endothelial cells. Findings revealed that 30% of LN patients had anti-C3 autoantibodies, which impaired negative complement regulators and heightened C3 deposition on endothelial cells, correlating with disease activity and potentially exacerbating autoimmune pathology by overactivating the complement system.
Reference
-
Vasilev, Vasil V., et al. "Functional characterization of autoantibodies against complement component C3 in patients with lupus nephritis." Journal of Biological Chemistry 290.42 (2015): 25343-25355. Distributed under Open Access license CC BY 4.0, without modification.
Related Product
For Research Use Only.
Related Sections:


 Fig.2 Effects and significance of anti-C3 antibodies.1
Fig.2 Effects and significance of anti-C3 antibodies.1