Product List Background C6 Functional Service
Background
The complement system, functioning as a ‘complementation’ of the immune system, enhances the ability of antibody-mediated cytotoxicity and phagocyte clearance of microorganisms and damaged cells through the formation of membrane attack complex (MAC). Complement C6 (C6) is one of the composed proteins of the MAC on the surface of pathogen cell membranes. Human C6 is a 934-amino acid glycoprotein composed of a single polypeptide chain, folding as nine auxiliary/regulatory domains complementing the MAC perforin core. C6 is the longest protein, as well as an essential constituent of the MAC. During the complement activation, the alpha chain of C5 is cleaved by C5 convertase releasing C5a and C5b. C5b is unstable and remains bound to the convertase complex for a short time until it binds to a C6 in the surrounding fluid to form C5b-6 complex. The C5b-6 complex can further bind complement components C7, C8, and C9, forming the MAC.
The current role of C6 is to participate in the formation of MAC, which plays critical functions in antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDCC). C6 deficiency is a rare autosomal recessive disorder. People with C6 deficiency are prone to bacterial infection (neisserial infections) and immunodeficiency due to a late component of complement deficiency.
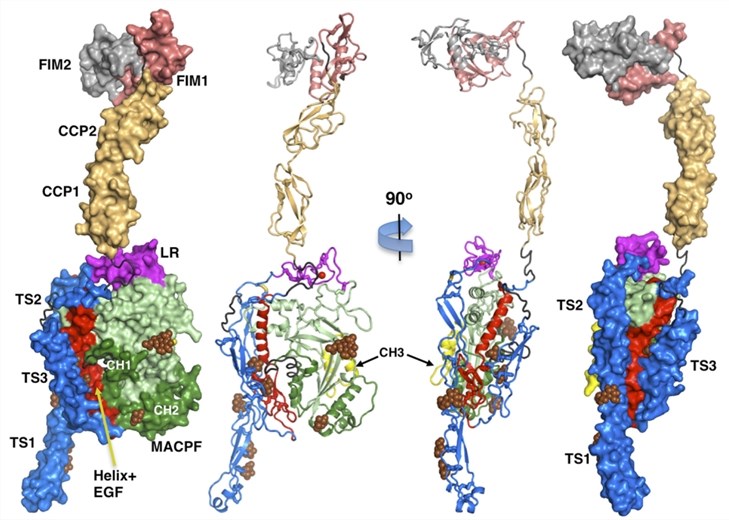 Fig.1 Crystal architecture and domain configuration of C6.1, 3
Fig.1 Crystal architecture and domain configuration of C6.1, 3
C6 Functional Service
Creative Biolabs provides an extensive range of C6-related products, which encompass anti-C6 antibodies, customized ELISA kits for C6, C6 proteins, peptides that block C6 and gene vectors encoding C6. These meticulously developed instruments are essential for driving research efforts focused on formulating therapeutic strategies for diverse diseases.
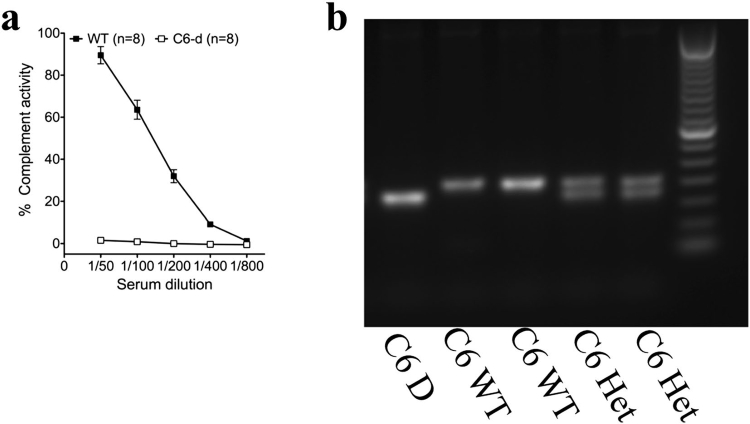 Fig.2 Renal biopsy findings in an aHUS patient before and after administration of anti-C5 monoclonal antibody therapy.2, 3
Fig.2 Renal biopsy findings in an aHUS patient before and after administration of anti-C5 monoclonal antibody therapy.2, 3
Researchers have investigated the involvement of the membrane attack complex in inducing lytic cell death and its role in secondary damage following CNS injury. Previous studies often used non-littermate control rats or mice with minimal C5b-9 activity. To elucidate C5b-9’s role in spinal cord injury and recovery, experiments were conducted using littermate rats that were either C6 wildtype or deficient. Genetic confirmation of C6 deficiency was performed alongside hemolytic analyses of total serum complement activity. The study evaluated functional and histological recovery following moderate contusion injury, comparing separate C6 WT and C6-D homozygous colonies with C6 WT and C6-D littermates derived from heterozygous colonies.
Creative Biolabs provides an array of tailor-made services centered on C6, encompassing thorough interaction analyses and supplementary functional features. These offerings are meticulously crafted to support clients in their esteemed scientific research and clinical activities.
References
-
Aleshin, Alexander E., et al. "Structure of Complement C6 Suggests a Mechanism for Initiation and Unidirectional, Sequential Assembly of Membrane Attack Complex (MAC)*♦." Journal of Biological Chemistry 287.13 (2012): 10210-10222.
-
Su, Diane, et al. "Complement C6 deficiency exacerbates pathophysiology after spinal cord injury." Scientific reports 10.1 (2020): 19500.
-
Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig.1 Crystal architecture and domain configuration of C6.1, 3
Fig.1 Crystal architecture and domain configuration of C6.1, 3
 Fig.2 Renal biopsy findings in an aHUS patient before and after administration of anti-C5 monoclonal antibody therapy.2, 3
Fig.2 Renal biopsy findings in an aHUS patient before and after administration of anti-C5 monoclonal antibody therapy.2, 3
