The human brain mainly receives external information through five senses: sight, hearing, smell, touch, and taste, of which at least 80% of external information is obtained through vision. The loss of vision not only brings many inconveniences to daily life, but also deals a heavy blow to the blind spiritually.
For a long time, it has been extremely difficult to treat blindness and there has been a lack of clinically effective methods to treat blindness. Many congenital and progressive blindnesses are associated with genetic mutations, and in recent years, with the maturation of gene therapy technology, more and more research has been conducted to treat blindness through gene therapy.
Leber Congenital Amaurosis (LCA), the earliest occurring and most severe inherited retinal disorder, is also one of the most common congenital blindness diseases, affecting approximately 1 in 40,000 newborns, with progressive loss of visual function in both eyes at birth or within one year after birth, resulting in congenital blindness in infants and children.
Recently, researchers at the University of Pennsylvania published a study in the Cell subjournal iScience titled “Night vision restored in days after decades of congenital blindness”. In this study, two patients who had been blind as children due to LCA, and who received experimental adeno-associated virus (AAV) gene therapy 20 to 30 years after their blindness, experienced an amazing restoration of night vision in just a few days.
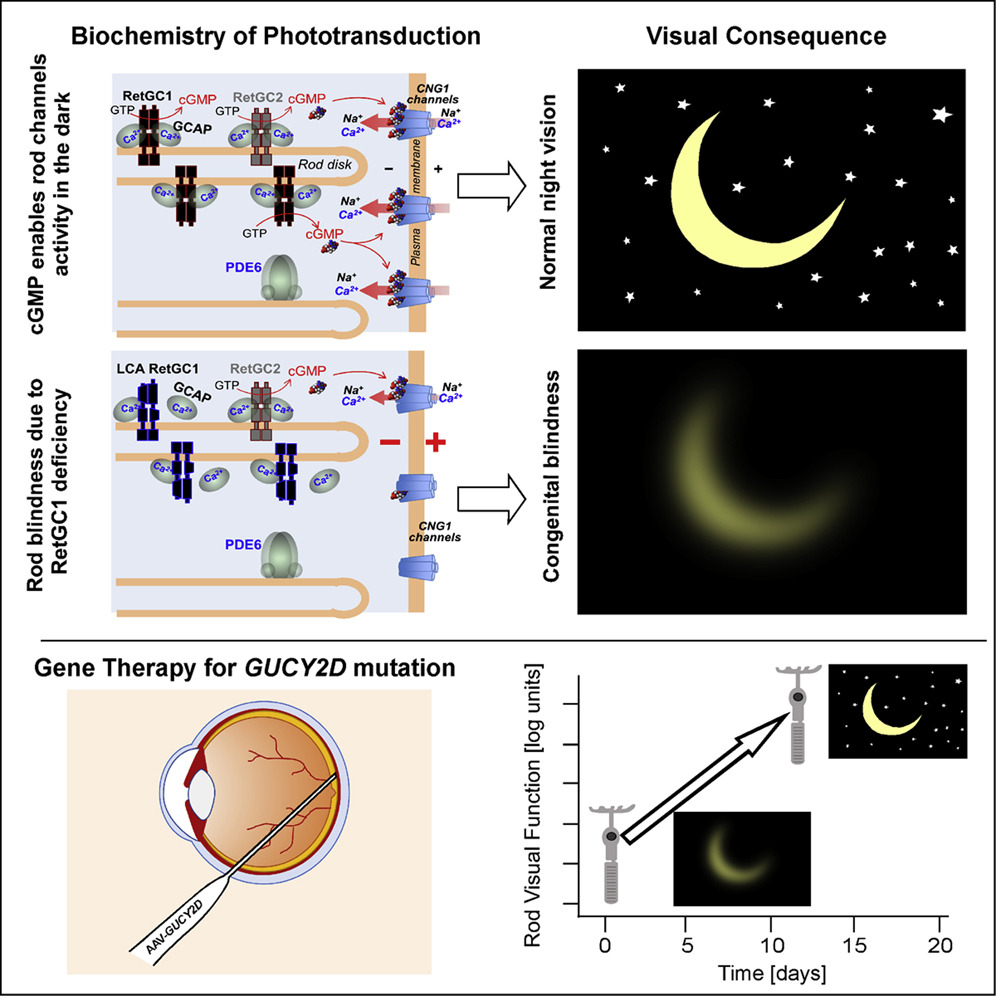
The two patients, who suffered from LCA caused by a mutation in the GUCY2D gene, had complete loss of night vision prior to treatment. The patients received an injection of an AAV vector carrying the correct version of the GUCY2D gene in one eye, and after a few days of treatment, the patient’s treated eye showed a dramatic increase in nighttime visual function and was approaching normal night vision.
LCA is mostly autosomal recessive, and 24 different types of congenital hemianopsia caused by genetic mutations have been identified. For example, in December 2017, the US Food and Drug Administration (FDA) approved Spark’s AAV gene therapy to treat LCA type 2 by delivering the correct RPE65 gene to retinal cells via an AAV vector, which sells for up to $850,000/year. In December 2018, Editas Medicine received FDA approval for CRISPR-Cas9 gene editing for the treatment of LCA type 10 caused by mutations in the CEP290 gene.
Up to 20 percent of patients with LCA are affected by mutations in the GUCY2D gene, which encodes a key protein required for the “phototransduction cascade” in retinal photoreceptor cells, a process that converts light signals into neuronal signals. Previous studies have shown that patients with LCA due to GUCY2D mutations often have some surviving photoreceptor cells, particularly in areas where optic rods (responsible for night vision) are abundant, suggesting that optic rod phototransduction can work again in the presence of GUCY2D.
In this study, the team used high doses of AAV gene therapy in two patients with LCA due to GUCY2D gene mutations. The two patients, a 19-year-old male and a 32-year-old female, had severely impaired vision as children. During the day when light is strong, their vision is severely deficient, and at night they are effectively blind, with less than one ten-thousandth the sensitivity to light of a normal person.
The research team treated only one eye of the patients, using the other untreated eye as a control to measure the effect of the treatment. Post-treatment tests showed that in both patients, the treated eye was thousands of times more sensitive to light in low-light conditions, essentially correcting the initial night vision deficit.
The researchers used nine complementary methods to measure the patients’ light sensitivity and functional visual acuity, including a test of room navigation ability in low-light conditions and a test of pupils’ involuntary response to light. The tests consistently showed significant improvements in optic rod cell-based vision in low light, and the patients’ daily lives improved, for example, they could now recognize objects and people in the dark.
In addition, surprisingly, the improvement of this gene therapy was very rapid, with two patients showing observable treatment effects after only 8 days of treatment.
The results of this study confirm AAV delivery of the GUCY2D gene restores photoreceptor function in optic rod cells, and also highlight a noteworthy fact—in some patients suffering from severe congenital vision loss, the retinal cell network that regulates vision remains largely active and intact, and only an additional supplementation of a missing protein is needed to get the retinal network working again and improve vision.
Dr. Samuel G. Jacobson, Professor of Ophthalmology at the University of Pennsylvania and the leader of the study, said these exciting results suggest that the basic molecular mechanisms of phototransduction remain largely intact in some patients with LCA, and, therefore, they can still be treated with gene therapy and receive improved vision even decades after blindness.
Reference
1. Jacobson, Samuel G., et al. “Night vision restored in days after decades of congenital blindness.” Iscience 25.10 (2022).
