Innovative gene therapy may hold the key to treating chronic pain in humans by reducing sodium ion levels, a recent study published in the international journal “Proceedings of the National Academy of Sciences” reveals. Researchers from institutions such as New York University have developed a novel gene therapy approach that indirectly regulates specific sodium ion channels, potentially alleviating chronic pain in individuals.
Chronic pain is a significant public health issue, affecting approximately one-third of the US population. Scientists have been eager to develop more effective and safer analgesics than opioid drugs. Sodium ion channels play a crucial role in the generation and transmission of pain because of their vital role in communication between nerve cells or neurons. A specific sodium ion channel known as NaV1.7 has emerged as a potential target for treating human pain, as previous research has demonstrated its importance in patients with rare genetic pain disorders.
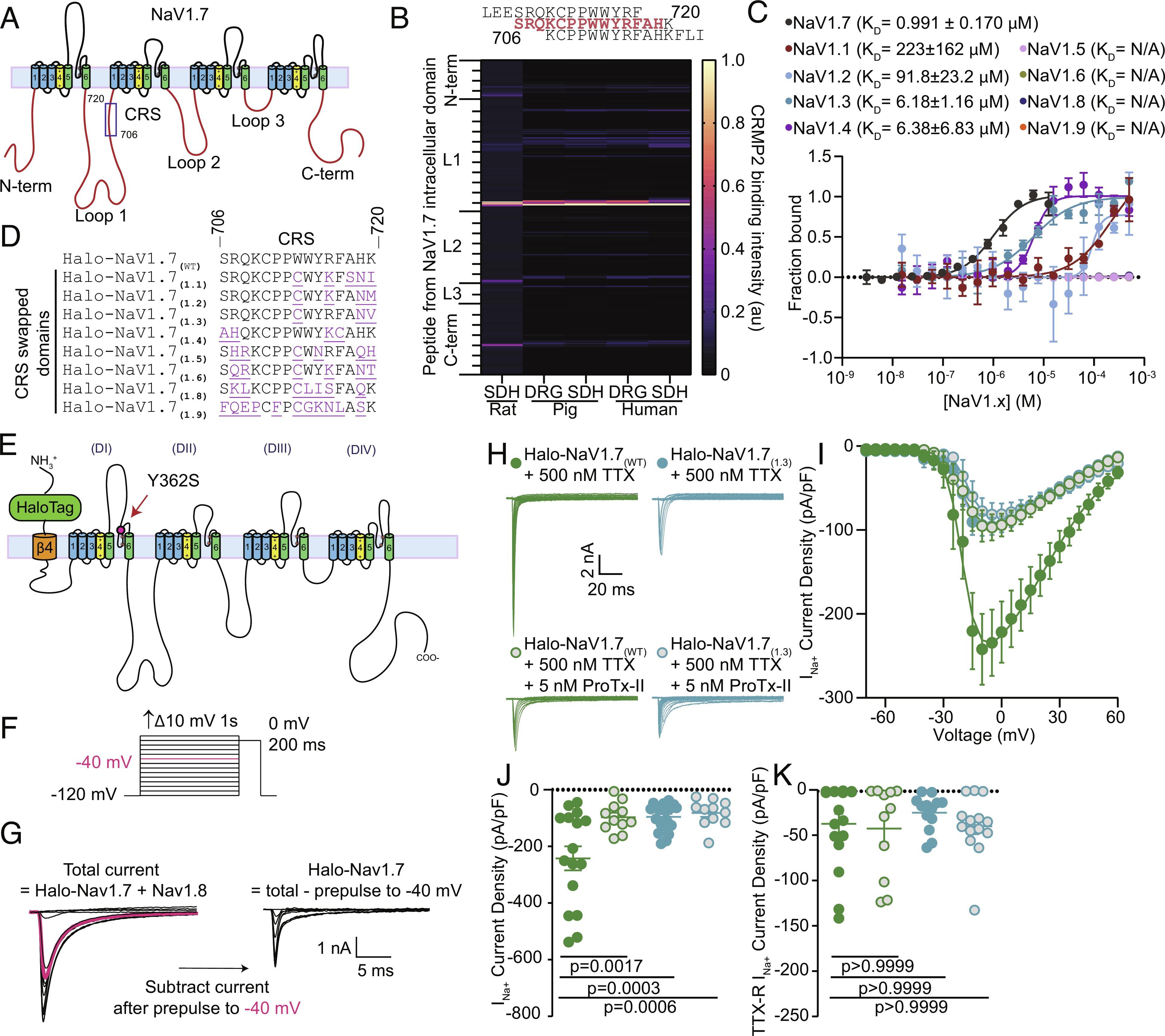
In some families, genetic mutations in the gene encoding NaV1.7 lead to an influx of sodium ions into cells, resulting in intense pain, while in other families, mutations that block NaV1.7 lead to complete painlessness. For years, scientists have been researching and attempting to develop new pain therapies that selectively block NaV1.7, but with limited success. Researchers like Khanna and colleagues took a different approach, not by blocking NaV1.7 directly but by modulating it through a protein called CRMP2.
According to Khanna, CRMP2 can interact with sodium ion channels and regulate their activity, allowing more or fewer sodium ions to enter the channels. By inhibiting the interaction between Nav1.7 and CRMP2, it may be possible to reduce sodium ion influx, calming down neurons and thereby reducing pain sensations in the body. Previously, researchers developed a small molecule that indirectly regulated Nav1.7 expression by targeting CRMP2. This compound successfully controlled pain in cell and animal models, and current research is exploring its application in humans.
While this compound has shown promise, a critical question remained: why does CRMP2 interact exclusively with NaV1.7 sodium ion channels and not with the other eight sodium ion channels in the same family? In this study, researchers identified a specific region in NaV1.7 where CRMP2 protein binds to regulate its activity. This region appears unique to NaV1.7, as CRMP2 does not directly bind to other sodium ion channels. This discovery raised excitement among researchers because removing this specific portion of the NaV1.7 channel would result in the loss of CRMP2’s regulatory effect.
To restrict the interaction between CRMP2 and NaV1.7, researchers created a peptide from the channel that corresponded to the region where CRMP2 binds. They then inserted this peptide into adenoviruses, which were used to transport it into neurons and inhibit the function of NaV1.7. The use of viruses to deliver genetic material into cells is a primary method in gene therapy, which has already been successful in treating blood disorders, eye conditions, and other rare diseases.
Researchers injected these engineered viruses into mice experiencing various forms of pain, including touch, heat, cold, and chemotherapy-induced peripheral neuropathy. After one to ten days, the researchers evaluated the animals and found that their pain sensations had reversed. Khanna stated that they have discovered a new approach that uses engineered viruses containing a small piece of genetic material that everyone possesses to infect neurons effectively, providing an effective treatment for pain. This represents a significant advancement in the field of gene therapy for chronic pain and is just one of the latest examples of its potential applications.
Researchers replicated their findings of NaV1.7 inhibition in multiple species, including rodents, primates, and human cells. While further research is needed, this study holds promise, indicating that their approach could potentially translate into therapeutic methods for humans. There is an urgent need to develop new pain therapies, especially for cancer patients suffering from chemotherapy-induced neuropathy. The long-term goal of researchers is to develop a novel gene therapy that can better treat these painful conditions and improve patients’ quality of life. In conclusion, the study’s results support the idea that the CRMP2 regulatory sequence (CRS) domain may be a targetable region for treating chronic neuropathic pain in humans.
Reference
1. Gomez, Kimberly, et al. “Identification and targeting of a unique NaV1. 7 domain driving chronic pain.” Proceedings of the National Academy of Sciences 120.32 (2023): e2217800120.
