Hepatocellular carcinoma (HCC), caused by increased obesity levels, poses a new health burden. However, there is currently a lack of optimal therapies for treating HCC. Tyrosine kinase inhibitors, including sorafenib, lenvatinib, regorafenib, and cabozantinib, have been used as first- or second-line treatments for unresectable liver cancer patients.
However, these drugs are associated with significant toxicity and poor quality of life outcomes, and their survival benefits are limited to a few months. Despite revolutionary advancements in immunotherapy for various types of cancer, the treatment outcomes for HCC remain suboptimal. Therefore, there is an urgent need to identify new treatment strategies for hepatocellular carcinoma.
Recently, researchers from the University of California published a study in the journal Molecular Therapy titled “miR-22 gene therapy treats HCC by promoting anti-tumor immunity and enhancing metabolism,” which reveals the therapeutic potential of MiR-22 gene therapy in treating liver cancer through promoting anti-tumor immunity and enhancing metabolism.
miR-22 is a microRNA that can be induced by beneficial metabolites with metabolic and immunological properties, including retinoic acid, bile acids, vitamin D3, and short-chain fatty acids. The tumor-suppressive role of miR-22 has been proposed, but its therapeutic potential in treating hepatocellular carcinoma (HCC) has not been determined. The role of miR-22 in regulating tumor immunity is also not well understood.
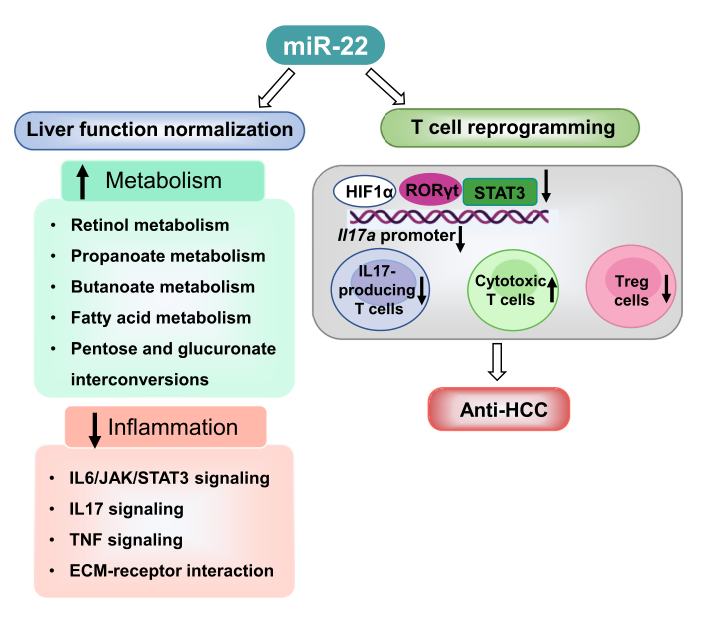
The study data demonstrates that miR-22 delivered by type 8 adenovirus effectively treated liver cancer. Compared to the FDA-approved lenvatinib, miR-22 produced better survival outcomes without significant toxicity. MiR-22 inhibited hypoxia-inducible factor 1 (HIF1a) and enhanced retinoic acid signaling in hepatocytes and T cells.
Furthermore, miR-22 therapy improved metabolism and reduced inflammation. In the liver, miR-22 reduced the abundance of IL-17-producing T cells by decreasing the occupancy of HIF1a at the RORC and IL17A genes and inhibited IL-17 signaling. Conversely, enhancing IL-17 signaling improved the anti-liver cancer effects of miR-22. Additionally, miR-22 expanded cytotoxic T cells and reduced regulatory T cells (Treg).
Furthermore, the anti-liver cancer effects of miR-22 were abolished when cytotoxic T cells were depleted. In patients, compared to miR-22-low HCC, miR-22-high HCC upregulated metabolic pathways and suppressed IL-17 pro-inflammatory signaling. In summary, miR-22 gene therapy could be a novel option for treating liver cancer.
Interestingly, the synthesis of retinoic acid in intestinal dendritic cells is controlled by aldehyde dehydrogenase ALDH1A, whose expression is induced by HDAC inhibitors such as butyrate and propionate, which are inducers of miR-22. Thus, there is an interaction between single-chain fatty acids and retinoic acid signaling.
Overall, this study demonstrates that the metabolic pathways of short-chain fatty acids and retinoic acid are decreased in hepatocellular carcinoma but induced after miR-22 treatment. The role of miR-22 in regulating the gut microbiota remains unclear. These data suggest that intertwined signaling pathways discovered in the gut impact liver health. Targeting these pathways along the gut-liver axis may provide new therapeutic options for treating liver cancer.
Reference
1. Hu, Ying, et al. “miR-22 gene therapy treats HCC by promoting anti-tumor immunity and enhancing metabolism.” Molecular Therapy (2023).
