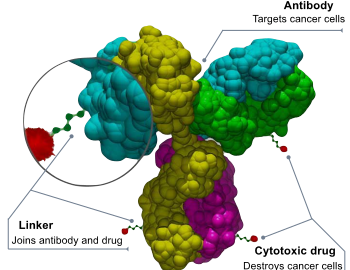
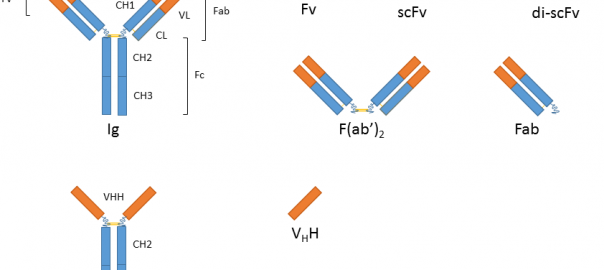
What Are the Development Prospects & Anticancer Potential of CD38 Antibodies?
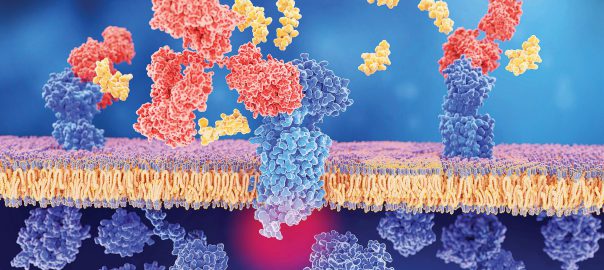
CD38 protein, which was discovered in 1980, is a type II transmembrane glycoprotein with a size of 46 kDa. The ligand of CD38 is CD31, also known as PECAM-1, which is aRead More…