What is antibody (conventional antibody)?
Before we begin to introduce recombinant antibody, we should learn about antibody. Antibody is known as immunoglobulins which are generally Y-shaped. They are peptide molecule secreted by b cells, mostly by differentiated B cells called plasma cells. Basically the function of antibodies is that control and stop pathogens and to assist in an immune response. They can protect us against infection and intoxication by mechanism of action for antibody functionality, such as antagonism, agonism, or cell killing via ADCC, ADCP, CDC, apoptosis PCD, or lysosomal-related PCD.
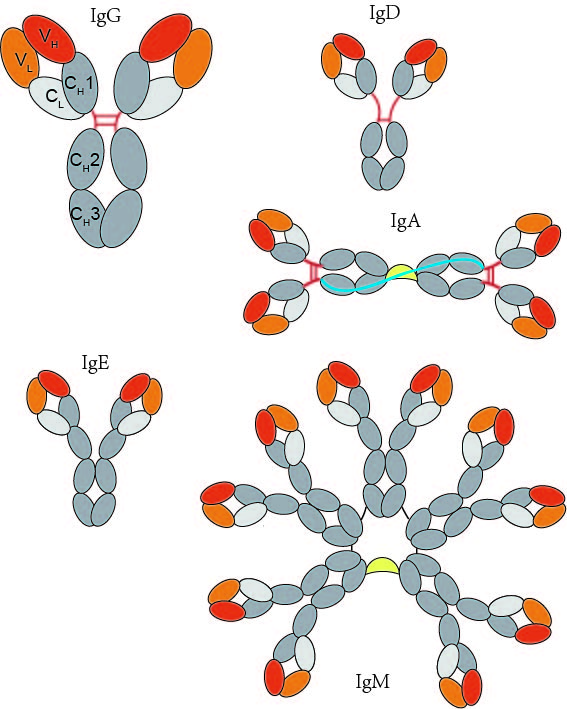
Much has been learned about the antibody structure (Fig. 1). IgG is a heterotetrameric protein assembled from two identical heavy and light chains (H, L), assembled by disulfide bonds. Each chain has two regions, the constant region (C) and the variable region (V). There are two parts on the right chain. The top is the variable region of the light chain (VL) and the bottom is the constant region of light chain (VH). Similarly, the heavy chain is divided into four parts. The top one-fourth of it is the variable region of the heavy chain and the other three parts are the constant regions (CH1, CH2, CH3). The “arms” of the “Y” bind antigens. The tail of the “Y” is responsible for biological activity, such as C’ activity or binding to cells. The antigen binding region also called paratope is a small part of variable region which recognizes and binds to an antigen. Each arm of the Y shape of an antibody monomer is tipped with a paratope, which is a set of complementarity determining regions that short for CDR.
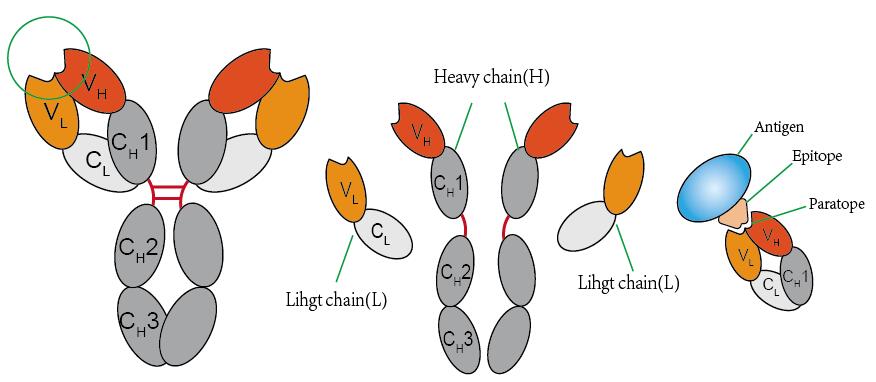
Fig. 1 Structure of Antibody (Immunoglobulins)
Antibody Isotypes
The constant region is identical in all antibodies of the same isotype, but differs in antibodies of different isotypes. The sequence of the heavy chain defines the class of Ig, such that α, δ, ε, γ and μ heavy chains define the immunoglobulins A (IgA), immunoglobulins D (IgD), immunoglobulins E (IgE), immunoglobulins G (IgG), and immunoglobulins M (IgM) classes, respectively, each with a distinct role in the human adaptive immune system (Fig. 2). The light chains are either kappa (κ) or lambda (λ) isoforms for all classes.
Fig. 2 Antibody Isotypes
Human IgA constitutes only 13% (2.1 mg/ml) of the antibody in human serum, but it is the predominant class of antibody in extravascular secretions. They are monmeric form in blood. The J chain is a 15-kD polypeptide consisting of 129 amino acid residues and one carbohydrate group. IgA1 is the most prevalent form in serum, but IgA2 is slightly more prevalent in secretions. Human IgE (190 kD) makes up less than 0.003% (0.4mg/ml) of the antibody in serum. IgE binds mast cells or basophils through its Fc part. IgE protects against parasites by releasing mediators that attract eosinophils. IgE is made up of about 13% carbohydrate. IgG is the most thoroughly studied in all five isotypes and recombinant antibody engineering is based on it. IgG constitutes about 80% (12.5 mg/ml) of the antibody in serum. Human IgG consists of four subclasses (isotypes), which are numbered in order of their serum concentrations (IgG1, IgG2, IgG3, and IgG4). The chief distinguishing characteristic among the four IgG subclasses is the pattern of inter chain linkages in the hinge region. IgM, primarily induced by polysaccharide antigens, is a 950-kD pentamer that makes up about 8% (1.25mg/ml) of the antibody in the serum. The five monomeric IgM molecules are arranged radially, the fab fragments pointing outward and the Fc fragments pointing to the center of the circle. IgM can quickly clump antigen and efficiently activate complement. IgM acts as one of the main receptors on the surface of mature B cells, along with IgD. When IgM is a surface receptor, it is in its monomeric form.
Recombinant Antibody (rAb) Definition
What are recombinant antibodies? Recombinant antibodies are antibody fragments generated by using recombinant antibody coding genes as a source and display technology, delivering high reproducibility, specificity and scalability. Unlike monoclonal antibodies (mAbs) which are produced using traditional hybridoma technologies, rAbs do not need hybridomas and animals in the production process if you only use synthetic genes.
Production of Recombinant Antibody
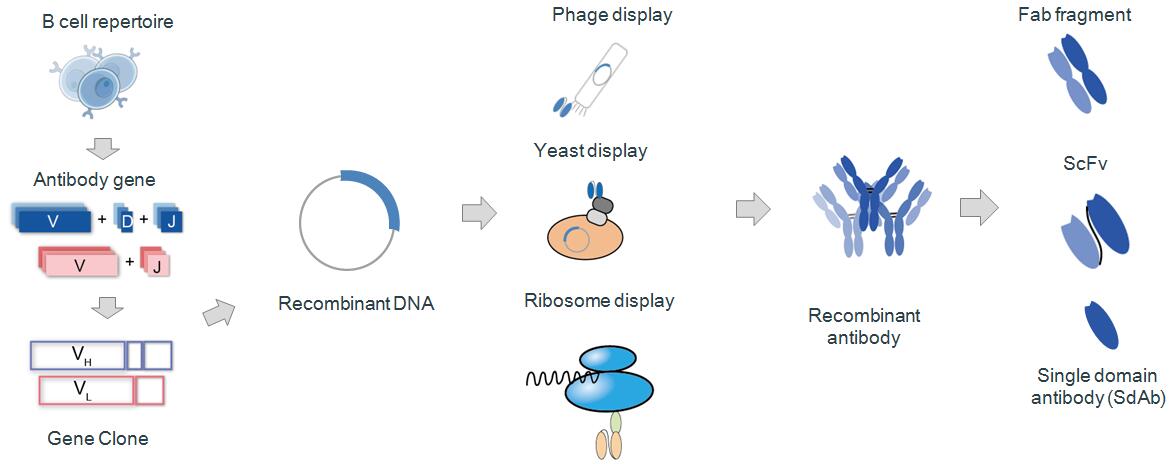
How is recombinant antibody produced? Let me show you the process of recombinant antibody production (Fig.3). As we know, antibody is composed of two heavy chains (VH) and two light chains (VL).
Step 1: Variable genes of heavy chain and light chain antibody should be cloned by PCR and designed primers. About the detailed strategy of PCR, scfv library construction protocol is introduced in resource of Creative Biolabs. By the recombinant DNA technology, link purified genes of VH and VL with prokaryotic expression vector which requires select in advance based on your target recombinant antibody fragments. If you need scfv fragment antibody, linker must be designed in your PCR strategy.
Step 2: Transformation: Electroporate ligation product of VH and VL received in previous step into cloned expression host cells. In this step, it is very important that highly competent of commercial source be recommended to obtain high transformation efficiency.
Step 3: Choose appropriate antibody display technology, such as phage display (which can screening small antibody fragment to obtain large antibody library); ribosome display (which can get a library of a capacity of 1014 without limitation of transformation efficiency and acquire mutant antibody library); yeast display (which make human antibody expression more superior because that yeast expression system is similar with mammalian cells). According to these above procedures, some recombinant antibodies can be constructed successfully, such as scfv antibody fragments, fab antibody fragments, and single domain antibodies.
Fig. 3 Production of Recombinant Antibody
Common Types of Recombinant Antibody
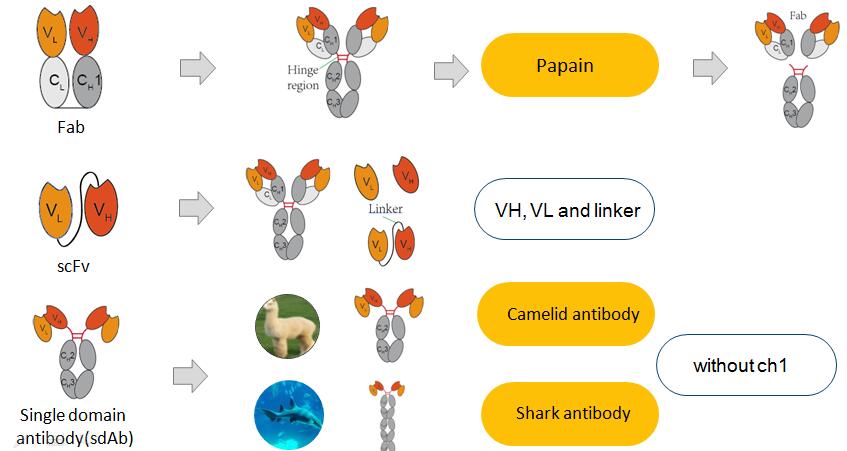
There are 3 common types of recombinant antibody fragments listed: Fab, scfv, single domain antibody (sdab) (Fig. 4).
Fab fragment was originally defined as one of the cleavage products after treatment of rabbit IgG with papain, which cleaves the core hinge, resulting in two identical fab fragments and the intact Fc as products. The molecular weight of Fab fragment is ~50 kDa. The nonspecific protease pepsin cuts below the first disulfide bond in the hinge region giving rise to a F(ab’)2 fragment. The molecular weight of F(ab’)2 fragment is ~100 kDa. The Fab contains four domains: the heavy chain variable domain (VH) linked to constant domain 1 (CH1), and the light chain variable domain (VL) linked to a constant domain (CL). Due to the hinge region which allows for flexibility of fabs in relation to the Fc, intact IgGs have proven difficult to crystallize in forms suitable for diffraction studies, and thus, to this time, only a few structures for intact IgGs have been determined.
Single-chain fragment variable (scfv) molecules combine the coding sequence of the variable heavy (VH) and sequence of the variable light chain (VL) domains of an antibody in a single-gene encoded format. The resulting polypeptides, with the variable light (VH ) and heavy chain (VH ) domains connected by a flexible peptide linker, were assemble into functional antigen-binding sites. The linker technology is a key step of success of constructing scfv antibody library. Scfvs have been developed as possible drug candidates in their own right, as well as components or domains of drug candidates.
Single domain antibody (sdAb) is discovered in in camelids and nurse sharks that consisted of a lone VH domain, lacking a paired VL, attached to a constant region. The primary advantages of domain antibodies as compared with scFvs are generally better folding and stability characteristics, the absence of the linker, and size.
Fig. 4 Common Types of Recombinant Antibody
Compared with conventional antibody, recombinant antibody (rAb) possesses some advantages, such as smaller size, monovalency, ease of engineering and manufacture, improved tissue penetration, no animal immunization and broader biodistribution, as well as lack of potentially deleterious Fc effector function. However, there are some drawbacks in recombinant antibodies, such as lower antibody yield, highly training and experienced technician. Most of scientists need to get them from outsourced companies because of complexity and intensive high technology of recombinant antibody production.
Application of Recombinant Antibody (rAb):
- desire or requirement for a short circulating half-life in serum;
- a smaller biologic that would have broader tissue distribution or the ability to penetrate tumors;
- a molecule that can be manufactured in either yeast or E. coli to potentially reduce cost of goods or increase scale of manufacturing;
- a molecule lacking an Fc effector functionality to eliminate both cellular responses against the target and potential for dimerization of receptors due to bivalency;
- a bispecific antibody fragment, such as has been demonstrated by BiTEs (bispecific T cell engagers; diabodies and most recently, DARTs;
- a molecule lacking an Fc effector functionality to eliminate both cellular responses against the target and potential for dimerization of receptors due to bivalency; a molecule that can be manufactured in either yeast or E. coli to potentially reduce cost of goods or increase scale of manufacturing.
Creative Biolabs is a professional custom service provider with extensive experience in antibody engineering. With over ten years of efforts, we have extended our services to over 26 countries. We provide recombinant antibody product, such as scfv antibody fragments, fab antibody fragments, single domain antibody, rabbit monoclonal antibodies, as well as recombinant antibody construction and expression services. If you are interested in learning more about how we can help with your projects, please don’t hesitate to contact us.