GPCR (G Protein-Coupled Receptor), the largest membrane protein family in mammalian genomes, is widely distributed in the central nervous system, immune system, cardiovascular system, and other organs and tissues, and participates in the development and normal function of the body. Once the regulation of the related intracellular pathway is abnormal, or the exogenous pathogens use it as receptors to attack the cells of the body, it will lead to a series of diseases. Therefore, GPCR is regarded as an important target for drug development, and the development of antibody drugs around members of the GPCR family is of great significance. However, only 108 of a total of 850 (human) GPCRs have been targeted. At the same time, obtaining highly efficient and selective small molecules remains a challenge, which creates new opportunities for the development of biological agents such as GPCR-binding antibodies and their fragments. In addition, GPCR exists in a variety of conformations, which makes it more challenging to find conformational conjugates.
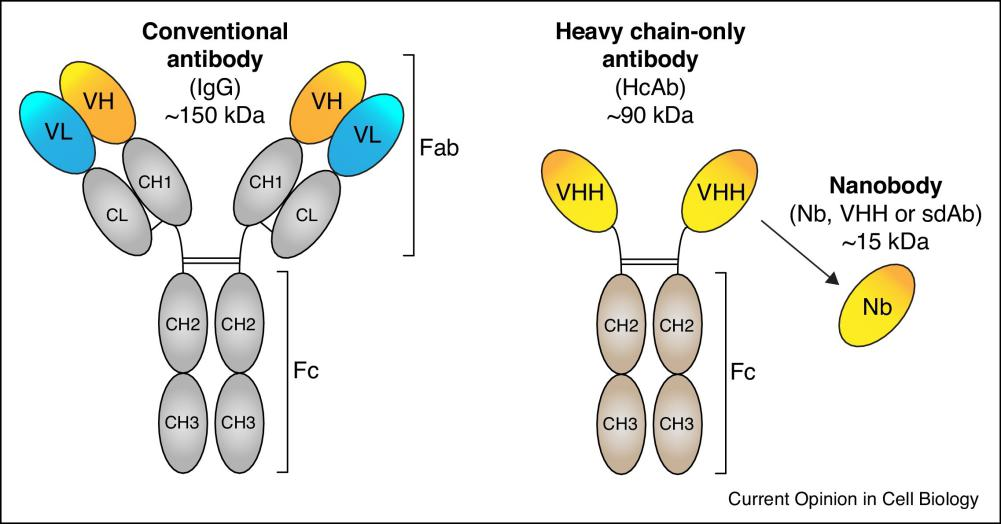
Fig.1 Diagram of Antibodies
Compared with traditional antibodies or Fab fragments, the small size and shape of single-domain antibodies allow them to bind to various GPCR rings at the same time, while the relatively long CDR of single-domain antibodies enables epitopes to bind between the transmembrane α-helix of the receptor. The convex shape of single-domain antibodies also allows them to bind to conformational epitopes, making them ideal candidates for targeting challenging antigens such as GPCRs. These favorable features of heavy-chain only antibodies, like the VHH domain, may be conducive to the generation of GPCR specific immune responses in vivo and the subsequent single-domain antibody selection process.
In the past decade, more and more GPCR-targeting single-domain antibodies against extracellular and intracellular epitopes have been developed. This single-domain antibody is developed for different applications, such as pharmacological regulation of receptor activity (antagonism, reverse activation), regulation or stabilization of receptor conformation, or the discovery of new small molecular drugs. Different forms of single-domain antibodies have been reported, including univalent single-domain antibodies, bivalent single-domain antibodies, biparatopic and bispecific single-domain antibodies.
The single-domain antibodies targeting GPCR can be divided into two types: extracellular binders and intracellular binders. With regard to single-domain antibodies binding to the extracellular domain of GPCR, most of these single-domain antibodies target peptidergic receptors, such as chemokine receptors. In addition to extracellular binding to single-domain antibodies, a variety of single-domain antibodies were developed for the intracellular part of GPCR. The initial study used the ability of these single-domain antibodies to recognize nonlinear conformational epitopes on GPCR. Interestingly, immunization and subsequent selection of receptors allow the development of conformational-specific single-domain antibodies in the presence of small molecular ligands (agonists, antagonists). This leads to the formation of agonist-bound GPCR crystal structure, which provides valuable information for the activation mechanism of GPCR and allows structure-based drug design.
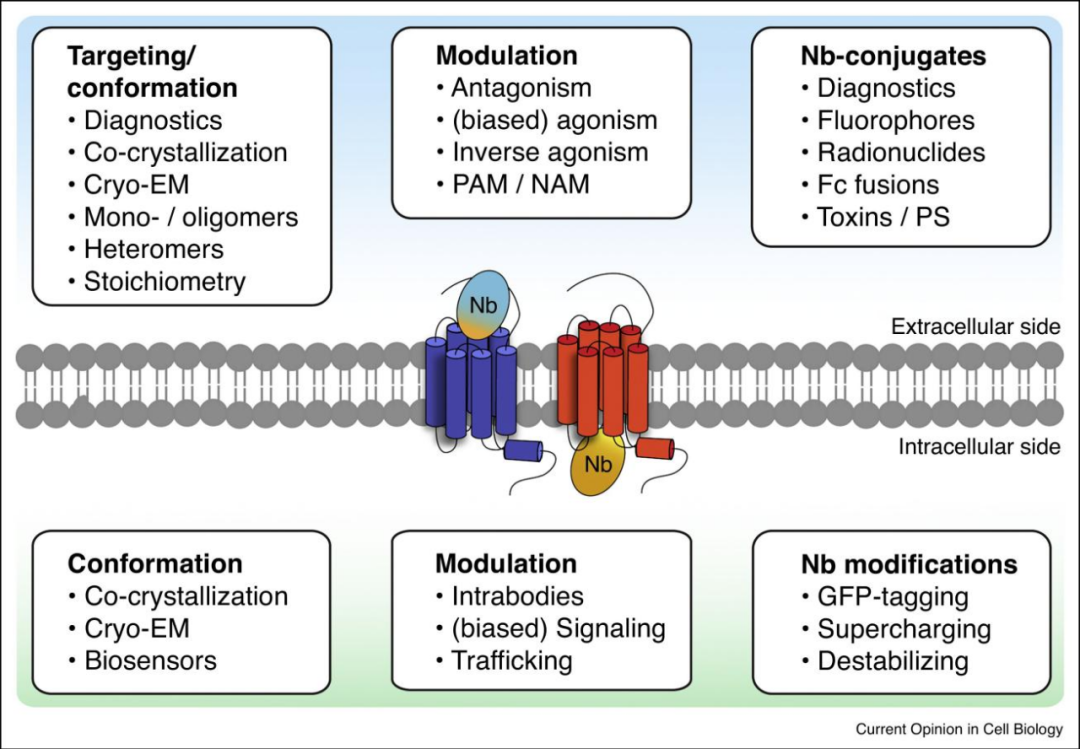
Fig.2 Current and potential applications of single-domain antibodies targeting GPCR from outside or inside cells
By stabilizing the conformation of the receptor inside the cell, single-domain antibodies can affect the affinity of ligands through allosteric regulation. Single-domain antibodies have emerged as valuable tools and treatments in the field of GPCR, but there are still challenges to be overcome. With regard to extracellular binding single-domain antibodies, more positive (reverse) activation and antagonistic single-domain antibodies are likely to be developed. It will be more challenging to identify single-domain antibodies that act as PAM/NAM. For example, compared with their receptors, the relatively large size and binding mechanism of chemokines will challenge the development of receptor binders/regulators that do not compete with ligand binding.
However, the identification of single-domain antibodies against class C GPCR with positive or negative allosteric regulation is more direct. Next, the role of GPCR monomers, homodimers, heterodimers and higher-order oligomers is still an interesting research field. So far, it has not been reported that single-domain antibodies can identify, stabilize, or prevent monomer/dimer/oligomer formation in an endogenous environment. It would be interesting to use formatted single-domain antibodies as a tool to further assess the importance of GPCR stoichiometry to GPCR function. The intracellular delivery of proteins, especially single-domain antibodies, has been widely studied, and some general methods include the use of gene therapy, (cationic) liposomes, nanoparticles and labeling with cell penetrating peptides. Interestingly, a relatively new method, also known as supercharging, involves mutating specific amino acids in the framework of single-domain antibodies, resulting in a high net charge of these single-domain antibodies, which allows single-domain antibodies to pass through the cell membrane. Although the mechanism behind this process and the clinical feasibility of this method are still unclear, it seems to be a promising method for therapeutic delivery targeting GPCR in vivo.
